eScore
abbvie.comThe eScore is a comprehensive evaluation of a business's online presence and effectiveness. It analyzes multiple factors including digital presence, brand communication, conversion optimization, and competitive advantage.
AbbVie maintains a high-authority digital presence with a corporate website that effectively serves as a hub for investors, partners, and talent. The content demonstrates strong domain authority in its core therapeutic areas and employs a sophisticated global-local structure for geographic reach. However, its direct engagement with patients and HCPs on the corporate site is more top-of-funnel, with a noticeable gap in unbranded, disease-state educational content that competitors are beginning to leverage.
Excellent content authority and a well-defined digital structure for key B2B stakeholders (investors, partners), reinforcing its position as an industry leader.
Develop comprehensive, unbranded 'Therapeutic Area Hubs' to capture significant organic search traffic from patients and HCPs, building early-stage trust and establishing market education leadership.
The company's messaging is highly consistent and effectively segmented, especially for B2B audiences like potential partners, where the communication is detailed and direct. The brand voice successfully balances scientific authority with a purpose-driven, compassionate tone. The primary weakness lies in an over-reliance on generic 'Learn More' CTAs and the lack of a clear, dedicated messaging pathway for Healthcare Professionals (HCPs) on the main corporate site.
Superb message tailoring for high-value audiences, particularly potential partners, with a clear and compelling value proposition for collaboration.
Replace generic 'Learn More' CTAs with specific, action-oriented language (e.g., 'Explore Our Pipeline') and create a dedicated 'For Healthcare Professionals' section on the homepage to better serve this critical audience.
The website provides a clean user experience with a logical information architecture and excellent mobile responsiveness, minimizing cognitive load. However, the conversion experience is hampered by subtle and aesthetically-driven call-to-action buttons that lack visual prominence, creating friction for key user goals like talent and partnership acquisition. The site is also described as static, missing opportunities for micro-interactions to enhance engagement.
A clear and intuitive navigation and information architecture that allows diverse user segments to find relevant information with minimal effort.
Increase the visual weight and contrast of all primary call-to-action buttons (e.g., 'Explore Careers', 'Submit an opportunity') to make them stand out and drive higher conversion rates.
AbbVie excels in establishing credibility through a best-in-class legal and compliance framework, featuring layered privacy notices and robust disclaimers. Third-party validation is consistently provided via news of positive clinical trials, strategic acquisitions, and detailed ESG reporting. The primary risk identified is not on the site itself but in the broader operational sphere, specifically regarding third-party vendor data security.
A mature, multi-layered privacy and compliance framework that not only mitigates legal risk but also serves as a powerful trust signal for all stakeholders.
Enhance transparency by creating a dedicated section that clearly outlines the company's approach to third-party vendor risk management and data security to reassure stakeholders.
AbbVie has demonstrated a remarkably sustainable competitive advantage by successfully navigating the Humira patent cliff, a monumental challenge. Its leadership in immunology is secured for the medium-term with the high-performing Skyrizi and Rinvoq platform, while aggressive M&A in oncology and neuroscience diversifies its moat. The primary disadvantage is the inherent high-risk, high-cost nature of pharmaceutical R&D.
A proven and highly effective strategy of combining a dominant commercial infrastructure with disciplined M&A to replace legacy revenue and build new, defensible market-leading positions.
Continue to invest in next-generation therapeutic platforms (e.g., RNA therapies, cell and gene therapy) to build the next competitive moat and ensure long-term innovation leadership.
The company's business model is inherently scalable, with high operating leverage and a global commercial infrastructure capable of launching new products worldwide. Expansion potential is extremely high, driven by a robust pipeline and an aggressive M&A strategy to enter new therapeutic areas like psychiatry and obesity. The main bottleneck to this scalability is the immense operational complexity of integrating large-scale acquisitions.
A powerful dual-engine for growth that combines internal R&D with a prolific M&A strategy, providing multiple avenues for market expansion and pipeline replenishment.
Develop a systematized, repeatable playbook for post-merger integration to accelerate synergy realization and reduce the operational drag from frequent, large-scale acquisitions.
AbbVie's business model is exceptionally coherent, with every strategic decision—from R&D focus to major acquisitions—clearly aligned with the central goal of diversifying revenue beyond Humira. The company is successfully transitioning its revenue base to new growth drivers, demonstrating strong strategic focus. This coherence is only slightly impacted by the high debt load from acquisitions, which places some constraints on capital allocation.
An unwavering strategic focus on executing the post-Humira pivot, demonstrated by disciplined resource allocation towards high-growth assets like Skyrizi, Rinvoq, and strategic acquisitions.
Implement a clear, multi-year plan to de-leverage the balance sheet to increase financial flexibility and reduce risk associated with its acquisition-heavy growth model.
AbbVie wields significant market power, evidenced by its leadership in immunology and its ability to command premium prices for innovative biologics. Its market influence is demonstrated by its capacity to shape treatment standards and execute an industry-leading pivot away from a historic blockbuster drug. While the Humira biosimilar entry represented a hit to market share, the rapid uptake of its successors shows the company's power to defend its position and redefine its revenue base.
Dominant market share and pricing power in the high-value immunology sector, which serves as the financial engine for strategic diversification and further market expansion.
Aggressively pursue and publish head-to-head clinical trial data for key growth products against competitors to solidify their best-in-class status and defend market share.
Business Overview
Business Classification
Research-Based Biopharmaceutical Company
Diversified Healthcare Products
Pharmaceuticals
Sub Verticals
- •
Immunology
- •
Oncology
- •
Neuroscience
- •
Aesthetics
- •
Eye Care
Mature
Maturity Indicators
- •
Established global market leader in several therapeutic areas.
- •
Significant annual revenues and large market capitalization.
- •
Navigating a critical business model evolution due to the patent expiry of its former blockbuster drug, Humira.
- •
Actively acquiring and integrating companies to fuel pipeline growth.
- •
Consistent history of paying and increasing dividends to shareholders.
Enterprise
Steady
Revenue Model
Primary Revenue Streams
- Stream Name:
Immunology Portfolio (Skyrizi & Rinvoq)
Description:Sales of next-generation biologic drugs Skyrizi and Rinvoq for treating various autoimmune diseases. This portfolio is the primary growth driver, designed to replace revenue from the former blockbuster Humira.
Estimated Importance:Primary
Customer Segment:Healthcare Providers (Rheumatologists, Gastroenterologists, Dermatologists) & Payers
Estimated Margin:High
- Stream Name:
Neuroscience Portfolio (Botox Therapeutic, Vraylar, etc.)
Description:Sales of treatments for neurological and psychiatric disorders, including Botox Therapeutic for conditions like chronic migraine and Vraylar for schizophrenia and bipolar disorder. This is a significant and growing revenue segment.
Estimated Importance:Secondary
Customer Segment:Healthcare Providers (Neurologists, Psychiatrists) & Payers
Estimated Margin:High
- Stream Name:
Oncology Portfolio (Imbruvica, Venclexta, Elahere)
Description:Sales of targeted therapies for various cancers, particularly hematologic (blood) cancers. Growth in this area is fueled by both in-house development and strategic acquisitions like ImmunoGen (for Elahere).
Estimated Importance:Secondary
Customer Segment:Healthcare Providers (Oncologists, Hematologists) & Payers
Estimated Margin:High
- Stream Name:
Aesthetics Portfolio (Botox Cosmetic, Juvederm)
Description:Sales of medical aesthetic products, primarily facial injectables, acquired through the Allergan acquisition. Provides portfolio diversification outside of traditional pharmaceuticals.
Estimated Importance:Tertiary
Customer Segment:Aesthetic Medicine Practitioners & Consumers
Estimated Margin:Medium
- Stream Name:
Humira (Adalimumab)
Description:Legacy sales of the blockbuster immunology drug. While still significant, revenue is declining rapidly due to biosimilar competition following its loss of exclusivity.
Estimated Importance:Declining/Legacy
Customer Segment:Healthcare Providers & Payers
Estimated Margin:Medium-Low (due to competition)
Recurring Revenue Components
- •
Chronic disease treatments requiring ongoing prescriptions (e.g., rheumatoid arthritis, psoriasis, Crohn's disease).
- •
Maintenance therapies for cancer and neurological disorders.
- •
Repeat aesthetic treatments (e.g., Botox Cosmetic).
Pricing Strategy
Value-Based & Negotiated Pricing
Premium
Opaque
Pricing Psychology
- •
Rebates and formulary negotiations with Pharmacy Benefit Managers (PBMs) and insurers.
- •
Patient assistance programs to mitigate out-of-pocket costs.
- •
Tiered pricing strategies in different global markets.
Monetization Assessment
Strengths
- •
Successfully transitioning revenue base from Humira to a new growth platform (Skyrizi/Rinvoq).
- •
Highly profitable, patent-protected products with high margins.
- •
Diversified portfolio across multiple distinct, high-need therapeutic areas.
Weaknesses
- •
Ongoing revenue erosion from Humira biosimilar competition places pressure on overall growth.
- •
High debt load resulting from large-scale acquisitions like Allergan.
- •
Vulnerability of pipeline to clinical trial failures or regulatory setbacks.
Opportunities
- •
Aggressive pipeline expansion through strategic M&A to enter new growth areas like psychiatry and novel therapeutic platforms (e.g., CAR-T).
- •
Label expansion for key growth products like Skyrizi and Rinvoq into new indications.
- •
Leveraging AI and machine learning to accelerate drug discovery and development.
Threats
- •
Intensifying competition from major pharmaceutical rivals and agile biotech firms.
- •
Increased government and public pressure on drug pricing and reimbursement globally.
- •
Potential for accelerated biosimilar uptake and deeper price erosion for Humira.
Market Positioning
Leadership in specific therapeutic categories driven by deep scientific expertise and best-in-class assets, supported by a powerful global commercial infrastructure.
Global leader in immunology, significant player in hematologic oncology, aesthetics, and neuroscience.
Target Segments
- Segment Name:
Healthcare Providers (Specialists)
Description:Physicians, specialists (e.g., rheumatologists, oncologists, neurologists), and key opinion leaders who prescribe AbbVie's medicines.
Demographic Factors
Board-certified in specific therapeutic areas
Practice in hospitals, specialty clinics, or academic medical centers
Psychographic Factors
- •
Value clinical efficacy and safety data
- •
Seek innovative treatments for complex diseases
- •
Influenced by peer-reviewed publications and medical conferences
Behavioral Factors
Prescribe based on clinical guidelines and patient outcomes
Engage with pharmaceutical sales representatives for product information
Pain Points
- •
Lack of effective treatments for patients with severe or refractory disease
- •
Managing treatment side effects and patient adherence
- •
Navigating complex reimbursement and prior authorization processes
Fit Assessment:Excellent
Segment Potential:High
- Segment Name:
Payers & Health Systems
Description:Insurance companies, pharmacy benefit managers (PBMs), national health systems, and large hospital networks that control drug access and reimbursement.
Demographic Factors
Large, national or regional organizations
Government or private entities
Psychographic Factors
Focused on cost-containment and budget predictability
Prioritize value, cost-effectiveness, and real-world evidence
Behavioral Factors
- •
Develop drug formularies to manage access
- •
Negotiate rebates and discounts with manufacturers
- •
Implement utilization management controls (e.g., prior authorization)
Pain Points
- •
High cost of specialty and biologic drugs
- •
Managing increasing pharmaceutical expenditures
- •
Demonstrating value and improved health outcomes to justify costs
Fit Assessment:Good
Segment Potential:Medium
- Segment Name:
Patients & Caregivers
Description:Individuals with serious, chronic diseases who are the end-users of AbbVie's medicines.
Demographic Factors
Varies by disease state (age, gender, etc.)
Often managing long-term health conditions
Psychographic Factors
- •
Seek improved quality of life and symptom relief
- •
Desire hope and new options for difficult-to-treat conditions
- •
Concerned about treatment costs and access
Behavioral Factors
Adherence to prescribed treatment regimens
Seek information from physicians, patient advocacy groups, and online resources
Pain Points
- •
Living with the physical and emotional burden of chronic illness
- •
Navigating a complex healthcare system
- •
Affording and accessing specialty medications
Fit Assessment:Excellent
Segment Potential:High
Market Differentiation
- Factor:
Scientific Leadership in Immunology
Strength:Strong
Sustainability:Sustainable
- Factor:
Global Commercialization & Market Access Expertise
Strength:Strong
Sustainability:Sustainable
- Factor:
Strategic & Disciplined M&A Strategy
Strength:Strong
Sustainability:Sustainable
- Factor:
Intellectual Property Portfolio (Patents)
Strength:Moderate
Sustainability:Temporary
Value Proposition
To discover and deliver innovative medicines and solutions that solve serious health issues and address the medical challenges of tomorrow, making a remarkable impact on people's lives.
Excellent
Key Benefits
- Benefit:
Transformative Patient Outcomes in Complex Diseases
Importance:Critical
Differentiation:Somewhat unique
Proof Elements
- •
Extensive clinical trial data (Phase I-IV)
- •
Regulatory approvals from FDA, EMA, and other global bodies
- •
Patient testimonials and real-world evidence
- Benefit:
Robust Pipeline of Novel Therapies
Importance:Critical
Differentiation:Somewhat unique
Proof Elements
- •
Publicly disclosed R&D pipeline with 90+ programs
- •
Significant annual investment in R&D
- •
Active business development and acquisition of new technologies
- Benefit:
Comprehensive Patient Support Programs
Importance:Important
Differentiation:Common
Proof Elements
- •
Financial assistance programs
- •
Nurse ambassador programs
- •
Disease education resources
Unique Selling Points
- Usp:
The Skyrizi/Rinvoq immunology platform, demonstrating superior efficacy and a strong safety profile in multiple indications.
Sustainability:Medium-term
Defensibility:Strong
- Usp:
The Botox franchise, a unique dual-engine asset spanning both therapeutic (neuroscience) and aesthetic markets.
Sustainability:Long-term
Defensibility:Strong
- Usp:
A proven ability to successfully execute a post-patent cliff strategy, transitioning a massive revenue base to new growth drivers.
Sustainability:Long-term
Defensibility:Moderate
Customer Problems Solved
- Problem:
Unmet medical need in severe, chronic autoimmune diseases.
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Limited treatment options for certain types of cancer.
Severity:Critical
Solution Effectiveness:Partial
- Problem:
Debilitating symptoms of neurological and psychiatric disorders.
Severity:Major
Solution Effectiveness:Partial
Value Alignment Assessment
High
AbbVie's focus on immunology, oncology, and neuroscience directly addresses areas with significant ongoing unmet medical needs and large, growing patient populations.
High
The company's value proposition of delivering innovative, effective treatments for serious diseases aligns perfectly with the primary needs of patients and prescribing specialists.
Strategic Assessment
Business Model Canvas
Key Partners
- •
Biotechnology companies (for licensing and acquisitions)
- •
Academic and research institutions
- •
Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs)
- •
Genmab (co-development partner for Epkinly)
Key Activities
- •
Research & Development (drug discovery, clinical trials)
- •
Strategic Mergers & Acquisitions
- •
Global Manufacturing and Supply Chain Management
- •
Regulatory Affairs and obtaining market approvals
- •
Commercialization (Marketing, Sales, Market Access)
Key Resources
- •
Intellectual Property (patent portfolio)
- •
Scientific and clinical development talent
- •
Global sales and marketing infrastructure
- •
State-of-the-art manufacturing facilities
- •
Strong balance sheet and cash flow to fund R&D and M&A
Cost Structure
- •
High Research & Development expenses
- •
Selling, General & Administrative (SG&A) costs, including marketing and sales force
- •
Cost of Goods Sold (COGS) for manufacturing
- •
Costs associated with acquisitions and licensing (upfront payments, milestones)
Swot Analysis
Strengths
- •
Strong and diversified portfolio of growth products (Skyrizi, Rinvoq, Vraylar, Botox) successfully offsetting Humira's decline.
- •
Proven expertise in R&D and commercial execution, particularly in immunology.
- •
Robust cash flow generation supporting dividends, R&D, and strategic M&A.
- •
Successful track record of large-scale M&A integration (e.g., Allergan).
Weaknesses
- •
Significant revenue decline from former blockbuster Humira due to biosimilar entry.
- •
High debt levels from past acquisitions, which could constrain future flexibility.
- •
Dependence on a concentrated set of products for the majority of revenue and growth.
Opportunities
- •
Further pipeline expansion into high-growth therapeutic areas via aggressive business development and acquisitions.
- •
Continued label expansions for Skyrizi and Rinvoq to maximize their market potential.
- •
Leveraging new technology platforms (AI, RNA therapies, protein degraders) to build the next wave of innovation.
- •
Entering the lucrative obesity market through strategic partnerships.
Threats
- •
Intense competition from other major pharmaceutical companies with overlapping pipelines.
- •
Global government initiatives aimed at controlling drug prices, such as the U.S. Inflation Reduction Act.
- •
Risk of clinical trial failures for key late-stage pipeline assets.
- •
Potential for faster-than-expected market erosion of legacy products.
Recommendations
Priority Improvements
- Area:
Debt Management
Recommendation:Develop a clear, multi-year plan to systematically de-leverage the balance sheet post-acquisition spree, improving financial flexibility and reducing interest expense.
Expected Impact:Medium
- Area:
Pipeline Acceleration
Recommendation:Continue to aggressively pursue mid-to-late stage assets through M&A and licensing, focusing on novel mechanisms of action to de-risk the future pipeline and ensure sustained long-term growth.
Expected Impact:High
- Area:
Operational Efficiency
Recommendation:Drive further operational synergies from the Allergan integration and other acquisitions to manage operating expenses and protect margins amidst pricing pressures and Humira's decline.
Expected Impact:Medium
Business Model Innovation
- •
Expand 'beyond the pill' digital health solutions to improve patient adherence and outcomes, creating stickier customer relationships.
- •
Invest in and integrate AI/ML platforms across the R&D value chain, from target identification to clinical trial optimization, to increase R&D productivity.
- •
Explore value-based contracting models with payers, where reimbursement is tied to clinical outcomes, to defend premium pricing and demonstrate product value.
Revenue Diversification
- •
Continue strategic M&A to enter new, adjacent therapeutic areas with high unmet needs, as demonstrated by the recent push into psychiatry.
- •
Build a presence in the high-growth obesity market through strategic licensing or acquisitions of differentiated assets.
- •
Expand the AbbVie Contract Manufacturing business to leverage manufacturing expertise and capacity as an additional revenue source.
AbbVie is successfully executing one of the most significant business model transitions in the pharmaceutical industry, navigating the patent cliff of Humira, once the world's best-selling drug. The company's strategy is centered on a pivot to a new, diversified growth platform led by its next-generation immunology assets, Skyrizi and Rinvoq. This transition is well underway, with the combined revenue of these two products projected to exceed Humira's peak sales, demonstrating a highly effective lifecycle management and R&D strategy.
The acquisition of Allergan has been a crucial, albeit costly, element of this evolution. It provided immediate diversification into the durable neuroscience and aesthetics markets, reducing the company's acute dependency on immunology. However, this has resulted in a highly leveraged balance sheet, a key risk that requires prudent management. AbbVie's operating model is heavily reliant on a dual engine of internal R&D excellence and an aggressive external innovation strategy, utilizing strategic partnerships, licensing, and large-scale M&A to continually replenish its pipeline. The company's recent acquisitions and partnerships in areas like oncology, psychiatry, and novel technology platforms underscore its forward-looking approach to sourcing future growth.
Key challenges remain, including intense competition, persistent drug pricing pressures from governments and payers, and the inherent risk of clinical development. However, AbbVie's strong commercial execution, robust cash flow, and proven ability to manage complex market dynamics position it well for sustained, steady growth. The primary strategic imperative for the next 3-5 years will be to flawlessly execute the commercial ramp-up of its current growth drivers while successfully advancing the next wave of high-potential pipeline assets into the market.
Competitors
Competitive Landscape
Mature
Oligopoly
Barriers To Entry
- Barrier:
High Research & Development (R&D) Costs
Impact:High
- Barrier:
Intellectual Property & Patent Protection
Impact:High
- Barrier:
Stringent Regulatory Hurdles (e.g., FDA Approval)
Impact:High
- Barrier:
Economies of Scale in Manufacturing and Distribution
Impact:High
- Barrier:
Established Brand Loyalty and Physician Relationships
Impact:Medium
Industry Trends
- Trend:
Rise of Biosimilars and Generics
Impact On Business:Significant revenue erosion for off-patent blockbuster drugs like Humira. Increases urgency to innovate and launch new protected products.
Timeline:Immediate
- Trend:
Integration of AI and Machine Learning in R&D
Impact On Business:Opportunity to accelerate drug discovery, optimize clinical trials, and reduce costs. A key area of competitive differentiation.
Timeline:Near-term
- Trend:
Focus on Personalized Medicine and Targeted Therapies
Impact On Business:Shifts R&D towards more specialized, high-value treatments, particularly in oncology. Requires investment in companion diagnostics and data analytics.
Timeline:Immediate
- Trend:
Increased Pricing Pressure and Regulatory Scrutiny
Impact On Business:Puts pressure on profit margins and necessitates demonstrating clear value and cost-effectiveness of new medicines to payers and governments.
Timeline:Immediate
- Trend:
Strategic Mergers & Acquisitions (M&A) to Fill Pipeline Gaps
Impact On Business:Acquiring smaller biotech firms with promising candidates is a primary strategy to sustain growth, as seen with AbbVie's recent acquisitions.
Timeline:Near-term
Direct Competitors
- →
Johnson & Johnson (Janssen)
Market Share Estimate:Top 5 Global Pharma
Target Audience Overlap:High
Competitive Positioning:Diversified healthcare giant with strongholds in pharmaceuticals, medical devices, and consumer health, emphasizing broad-based innovation.
Strengths
- •
Highly diversified revenue streams across multiple healthcare sectors.
- •
Strong portfolio of blockbuster drugs, including Stelara in immunology and Darzalex in oncology.
- •
Extensive global commercial and distribution network.
- •
Significant R&D investment and a history of successful drug development.
Weaknesses
- •
Facing patent expiration for key drugs like Stelara, creating revenue pressure similar to AbbVie's Humira situation.
- •
Large size can sometimes lead to slower, more bureaucratic decision-making compared to more focused biopharma companies.
- •
Ongoing litigation risks related to various products.
Differentiators
Cross-segment synergies between pharmaceuticals, med-tech, and consumer health.
Leader in multiple myeloma treatments.
- →
Bristol Myers Squibb (BMS)
Market Share Estimate:Top 10 Global Pharma
Target Audience Overlap:High
Competitive Positioning:A leader in oncology and immunology, focused on innovative medicines for serious diseases.
Strengths
- •
Dominant position in immuno-oncology with blockbusters like Opdivo.
- •
Strong cardiovascular franchise with Eliquis (co-marketed with Pfizer).
- •
Successful strategic acquisition of Celgene, which significantly boosted its oncology and hematology pipeline.
- •
Robust R&D pipeline in key therapeutic areas.
Weaknesses
- •
High dependency on a few key products, making it vulnerable to patent cliffs and competition.
- •
Faces intense competition in the crowded immuno-oncology space from Merck and Roche.
- •
Dependence on the U.S. market for a large portion of its sales.
Differentiators
Pioneering leadership in immuno-oncology.
Strong expertise in cardiovascular medicines.
- →
Amgen
Market Share Estimate:Top 15 Global Pharma
Target Audience Overlap:High
Competitive Positioning:A leading biotechnology pioneer focused on developing innovative therapies for serious illnesses.
Strengths
- •
Strong and diverse portfolio of biologic drugs in areas like oncology, bone health, and cardiovascular disease.
- •
Robust pipeline with promising candidates in areas like obesity (MariTide).
- •
Deep expertise in biotechnology and large-molecule manufacturing.
- •
One of the first companies to launch a biosimilar to Humira (Amjevita), directly competing with AbbVie.
Weaknesses
- •
Faces significant patent expiration challenges for key drugs like Enbrel and Prolia.
- •
High R&D expenditure with pressure to deliver consistent blockbusters.
- •
Revenue concentration on a few key products creates vulnerability.
Differentiators
Biotechnology-first approach and legacy.
Dual position as both an innovator and a major biosimilar competitor.
- →
Pfizer
Market Share Estimate:Top 5 Global Pharma
Target Audience Overlap:High
Competitive Positioning:Global pharmaceutical leader with a broad, diversified portfolio and massive scale in R&D and commercialization.
Strengths
- •
Vast and diverse product portfolio across numerous therapeutic areas.
- •
Unmatched global scale in manufacturing, marketing, and distribution.
- •
Proven ability to rapidly develop and commercialize groundbreaking products (e.g., COVID-19 vaccine and treatment).
- •
Strong financial position enabling significant investment in R&D and strategic acquisitions.
Weaknesses
- •
Facing significant revenue decline from its COVID-19 franchise.
- •
Like peers, faces ongoing patent expirations on key drugs.
- •
Can be perceived as less focused than competitors specializing in specific therapeutic areas.
Differentiators
Industry-leading scale and global reach.
Strong expertise in vaccine technology (mRNA).
Indirect Competitors
- →
Biosimilar Manufacturers (e.g., Sandoz, Viatris, Teva)
Description:Companies that develop and market lower-cost, highly similar versions of biologic drugs once their patents expire. They compete directly on price and formulary access.
Threat Level:High
Potential For Direct Competition:They are already direct competitors for off-patent biologics like Humira. Their primary business model is to compete with innovator products.
- →
Emerging Biotechnology Companies
Description:Smaller, agile R&D-focused companies pioneering novel therapeutic platforms (e.g., gene editing, RNAi, cell therapies) that could disrupt existing treatment paradigms in AbbVie's core areas.
Threat Level:Medium
Potential For Direct Competition:High, as they are primary acquisition targets for large pharma but could also grow into direct competitors if they choose to commercialize their own products.
- →
Digital Therapeutics (DTx) Companies
Description:Companies developing software-based interventions to prevent, manage, or treat medical conditions. They can supplement or, in some cases, replace the need for traditional pharmaceuticals.
Threat Level:Low
Potential For Direct Competition:Low, more likely to become partners. However, in areas like neuroscience, DTx for depression or anxiety could reduce the market for pharmacological treatments.
Competitive Advantage Analysis
Sustainable Advantages
- Advantage:
Leadership in Immunology
Sustainability Assessment:Despite the Humira patent cliff, AbbVie has successfully transitioned its leadership to next-generation blockbusters Skyrizi and Rinvoq, which are projected to generate massive revenues and have long patent lives.
Competitor Replication Difficulty:Hard
- Advantage:
Strong Global Commercial Infrastructure
Sustainability Assessment:Decades of experience, established relationships with healthcare providers and payers, and a massive global salesforce are difficult to replicate and provide a significant advantage in launching new drugs.
Competitor Replication Difficulty:Hard
- Advantage:
Diversified Portfolio through Allergan Acquisition
Sustainability Assessment:The acquisition of Allergan provided durable, market-leading assets in aesthetics (Botox) and neuroscience, diversifying revenue away from immunology and creating new growth platforms.
Competitor Replication Difficulty:Hard
Temporary Advantages
- Advantage:
Market Exclusivity for Newly Launched Drugs
Estimated Duration:10-15 years (Patent Life)
Description:The patent protection on newly launched drugs and indications provides a temporary monopoly, allowing for premium pricing and market share capture before competitors can launch similar mechanisms or biosimilars enter.
Disadvantages
- Disadvantage:
Humira Patent Expiration and Revenue Erosion
Impact:Critical
Addressability:Moderately
Description:The loss of exclusivity for Humira, once the world's best-selling drug, creates a massive revenue hole that the company is actively working to fill with its newer portfolio. The speed and depth of biosimilar erosion is a major challenge.
- Disadvantage:
High R&D Costs and Pipeline Risk
Impact:Major
Addressability:Difficult
Description:Like all major pharma companies, AbbVie faces immense costs and high failure rates in drug development. A significant clinical trial failure for a late-stage asset could negatively impact future growth prospects.
Strategic Recommendations
Quick Wins
- Recommendation:
Aggressively defend and expand market share for Skyrizi and Rinvoq.
Expected Impact:High
Implementation Difficulty:Easy
Description:Maximize the commercial execution for these key growth drivers to offset Humira's decline as quickly as possible, focusing on new indications and head-to-head competitive data.
- Recommendation:
Optimize digital marketing and patient engagement programs.
Expected Impact:Medium
Implementation Difficulty:Moderate
Description:Leverage digital channels for more personalized HCP engagement and enhance patient support services to improve adherence and build loyalty, creating a competitive advantage beyond the drug itself.
Medium Term Strategies
- Recommendation:
Continue strategic, bolt-on M&A to acquire promising mid-to-late stage assets.
Expected Impact:High
Implementation Difficulty:Difficult
Description:Focus on acquiring innovative companies in core therapeutic areas (oncology, neuroscience) to de-risk the internal pipeline and add new growth drivers, as evidenced by recent deals.
- Recommendation:
Expand presence in high-growth emerging markets.
Expected Impact:Medium
Implementation Difficulty:Moderate
Description:Develop tailored commercial strategies to capitalize on the growing demand for advanced medicines in markets like China and Latin America.
Long Term Strategies
- Recommendation:
Invest in next-generation therapeutic platforms.
Expected Impact:High
Implementation Difficulty:Difficult
Description:Build or acquire capabilities in cutting-edge areas like cell and gene therapy, targeted protein degradation, or AI-driven drug discovery to ensure long-term competitiveness and build the next wave of innovative medicines.
- Recommendation:
Diversify further into adjacent healthcare areas.
Expected Impact:Medium
Implementation Difficulty:Difficult
Description:Explore opportunities in areas like digital health, diagnostics, or data analytics that complement the core pharmaceutical business and create new, sustainable revenue streams.
Solidify AbbVie's identity as the market leader in immunology, powered by next-generation therapies, while establishing itself as a top-tier player in oncology and neuroscience through strategic pipeline development and acquisitions.
Differentiate through best-in-class clinical data, a patient-centric approach that integrates support services with therapeutics, and operational agility in identifying and integrating external innovation.
Whitespace Opportunities
- Opportunity:
Develop a 'Beyond the Pill' ecosystem for immunology patients.
Competitive Gap:Most competitors focus primarily on the drug itself. A comprehensive digital platform for disease management, telehealth, and wellness could create significant patient and physician loyalty.
Feasibility:Medium
Potential Impact:High
- Opportunity:
Become a leader in AI-powered drug discovery for neurodegenerative diseases.
Competitive Gap:While many are exploring AI, few have established clear leadership. Focusing AI efforts on a complex, high-need area like Alzheimer's or Parkinson's could create a significant long-term advantage.
Feasibility:Low
Potential Impact:High
- Opportunity:
Expand the aesthetics portfolio into adjacent regenerative medicine.
Competitive Gap:The aesthetics market is moving towards more regenerative and less invasive solutions. Leveraging the Botox brand and infrastructure to move into this space could capture a new, high-growth market.
Feasibility:Medium
Potential Impact:Medium
- Opportunity:
Strategic partnerships in digital diagnostics.
Competitive Gap:Early and accurate diagnosis is key for many of AbbVie's therapeutic areas. Partnering with or acquiring digital diagnostic companies could help identify patient populations earlier, driving drug adoption.
Feasibility:High
Potential Impact:Medium
AbbVie operates within the mature, oligopolistic biopharmaceutical industry, which is characterized by intense competition, high barriers to entry, and a constant race for innovation. The company's primary competitive challenge is navigating the monumental patent cliff for its historic blockbuster, Humira. The entry of multiple biosimilars is already eroding this crucial revenue stream, a threat AbbVie has proactively addressed by developing and successfully launching its next-generation immunology drugs, Skyrizi and Rinvoq. This strategic pivot is central to maintaining its market leadership in immunology.
The competitive landscape is dominated by other large, well-funded pharmaceutical giants like Johnson & Johnson, Bristol Myers Squibb, Amgen, and Pfizer. These companies compete directly with AbbVie across its core therapeutic areas of immunology, oncology, and neuroscience, often with their own blockbuster drugs and robust R&D pipelines. The key battleground is not just product efficacy but also market access, physician relationships, and the speed of pipeline development.
AbbVie's primary sustainable advantages are its entrenched leadership and deep expertise in immunology, a powerful global commercialization engine, and a more diversified portfolio following the strategic acquisition of Allergan. This deal provided durable, non-correlated assets like Botox, reducing the company's dependency on its immunology franchise. However, the company's key vulnerability remains its success being tied to a small number of very high-revenue products, making pipeline execution and successful business development paramount.
Indirect competition from agile biotech firms and price pressure from biosimilar manufacturers represent ongoing threats. To counter this, AbbVie's strategy of acquiring external innovation, as highlighted by recent acquisitions on its website, is critical. Opportunities for differentiation lie in leveraging AI to accelerate R&D, building integrated patient support ecosystems that go 'beyond the pill,' and expanding into new high-growth technology platforms. Ultimately, AbbVie's future success will depend on its ability to execute its post-Humira growth strategy, continue to successfully integrate new acquisitions, and maintain a highly productive R&D pipeline to stay ahead of its formidable competitors.
Messaging
Message Architecture
Key Messages
- Message:
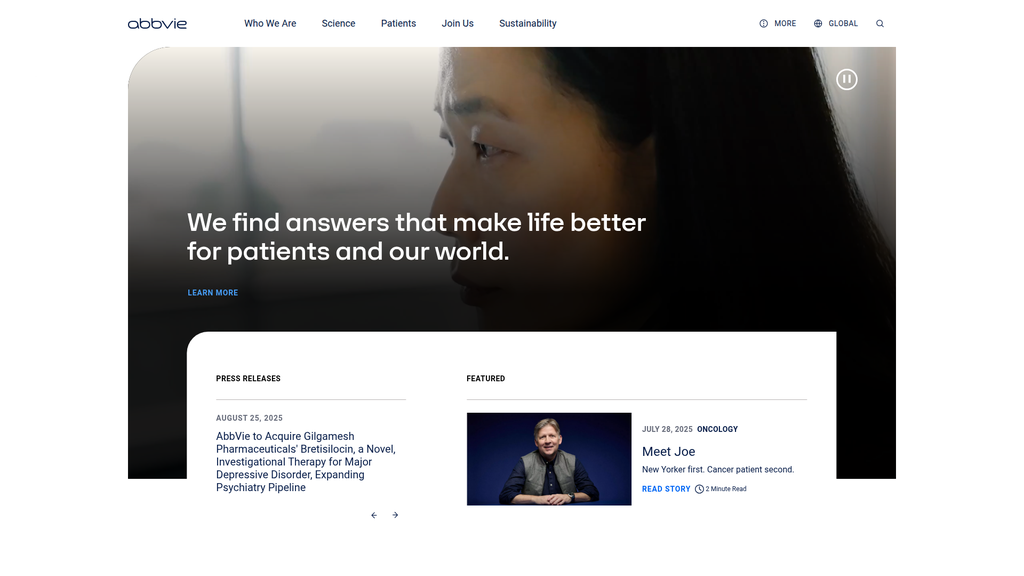
We find answers that make life better for patients and our world.
Prominence:Primary
Clarity Score:High
Location:Homepage Hero Banner
- Message:
Patients are at the heart of everything we do.
Prominence:Secondary
Clarity Score:High
Location:Patient Stories Section (Homepage)
- Message:
We solve the tough challenges.
Prominence:Secondary
Clarity Score:High
Location:Science & Innovation Section (Homepage)
- Message:
Innovation Takes All of Us.
Prominence:Primary
Clarity Score:High
Location:Partner with Us Page Hero
- Message:
We make an impact that lasts.
Prominence:Tertiary
Clarity Score:Medium
Location:ESG Section (Homepage)
The message hierarchy is logical and well-structured. The primary message on the homepage is broad and patient-focused, effectively setting a human-centric frame. Secondary messages then logically segment this core idea into key pillars: patient stories, scientific innovation, and corporate culture. The 'Partner with Us' page demonstrates excellent message tailoring with its own primary, collaborative message.
Messaging is highly consistent across the analyzed pages. Core themes of patient-centricity, scientific rigor, collaboration, and making an 'impact' are woven throughout the content, from high-level brand statements to specific descriptions of partnership approaches. The corporate mission of discovering and delivering innovative medicines is a constant undercurrent.
Brand Voice
Voice Attributes
- Attribute:
Purpose-Driven
Strength:Strong
Examples
- •
We find answers that make life better for patients and our world.
- •
Patients are at the heart of everything we do.
- •
Leading with purpose, we are willing to make the tough choices that deliver a lasting impact...
- Attribute:
Scientific & Authoritative
Strength:Strong
Examples
- •
250+ active external innovation partners to conduct groundbreaking science...
- •
Our core R&D areas of interest include immunology, oncology, neuroscience...
- •
We are committed to achieving long-term control and potential cure, using oral small molecules, targeted biologics...
- Attribute:
Collaborative
Strength:Strong
Examples
- •
Innovation Takes All of Us.
- •
We work with you as one connected unit to fuel scientific progress.
- •
We collaborate across disciplines to connect people and ideas...
- Attribute:
Compassionate & Human-centric
Strength:Moderate
Examples
- •
Meet Joe. New Yorker first. Cancer patient second.
- •
Explore the real life stories that inspire us...
- •
Inspired by the people behind the medicine we make.
Tone Analysis
Inspirational
Secondary Tones
- •
Professional
- •
Empathetic
- •
Confident
Tone Shifts
The tone shifts from broadly inspirational and patient-focused on the homepage to highly specific, technical, and business-oriented on the 'Partner with Us' page. This is an appropriate and effective shift based on the target audience.
Voice Consistency Rating
Excellent
Consistency Issues
No itemsValue Proposition Assessment
AbbVie combines tenacious, groundbreaking science with a deeply human-centric focus to discover and deliver innovative medicines that solve serious health issues and create a remarkable impact on patients' lives.
Value Proposition Components
- Component:
Scientific Leadership & Innovation
Clarity:Clear
Uniqueness:Somewhat Unique
- Component:
Patient-Centered Approach
Clarity:Clear
Uniqueness:Common
- Component:
Collaborative Partnership Model
Clarity:Clear
Uniqueness:Somewhat Unique
- Component:
Broad Therapeutic Expertise
Clarity:Clear
Uniqueness:Common
- Component:
Commitment to ESG
Clarity:Somewhat Clear
Uniqueness:Common
While many pharmaceutical companies claim patient-centricity and innovation, AbbVie's differentiation comes from the emphasis on 'solving tough challenges' and a clearly articulated, highly structured partnership model. The 'Partner with Us' page is a significant differentiator, moving beyond generic statements to provide concrete areas of interest and team structures, signaling a serious and organized approach to external innovation. The brand attempts to differentiate by highlighting the tenacity and curiosity of its people.
The messaging positions AbbVie as a major, established leader in the biopharmaceutical industry, on par with competitors like Pfizer, Merck, and Johnson & Johnson. The focus on a wide range of therapeutic areas—from immunology to aesthetics—communicates a diversified and resilient business model. The messaging strategy aims to build a reputation of a compassionate innovator, balancing the corporate scale with a focus on individual patient stories to build trust and soften the corporate image.
Audience Messaging
Target Personas
- Persona:
Potential Partners (Biotech, Academia)
Tailored Messages
- •
Innovation Takes All of Us.
- •
We have a highly integrated approach to developing and sustaining our partnerships.
- •
Detailed lists of 'Areas of interest' including specific diseases and technology platforms.
Effectiveness:Effective
- Persona:
Patients & Caregivers
Tailored Messages
- •
Patients are at the heart of everything we do.
- •
Explore the real life stories that inspire us...
- •
New Yorker first. Cancer patient second.
Effectiveness:Somewhat Effective
- Persona:
Investors
Tailored Messages
- •
AbbVie Reports Second-Quarter 2025 Financial Results
- •
We invest in creating better futures.
- •
Links to Events, Stakeholder Contacts, and Annual Reports.
Effectiveness:Effective
- Persona:
Potential Employees (Talent)
Tailored Messages
- •
a culture of curiosity
- •
Grow and fulfill your unique potential in our supportive environment...
- •
If you thrive as part of a diverse, collaborative team—we’re ready for you.
Effectiveness:Effective
Audience Pain Points Addressed
The uncertainty and emotional burden of serious illness (addressed through inspirational patient stories).
The difficulty for smaller biotech firms in finding a structured, collaborative, and well-resourced pharma partner (addressed on the 'Partner with Us' page).
Audience Aspirations Addressed
- •
The hope for new, better treatments for complex diseases.
- •
The desire for investors to see sustainable growth and a strong R&D pipeline.
- •
The ambition of scientists and professionals to work at an innovative company with a strong culture and purpose.
Persuasion Elements
Emotional Appeals
- Appeal Type:
Hope & Inspiration
Effectiveness:High
Examples
We find answers that make life better for patients and our world.
The patient story of 'Joe,' which humanizes the fight against cancer and focuses on his identity beyond the disease.
- Appeal Type:
Pride & Purpose
Effectiveness:Medium
Examples
Tenacity is the anchor of solving any real challenge. It's what keeps you there...
employees turning possibilities into reality for people and patients
Social Proof Elements
- Proof Type:
Scale & Reach (Metrics)
Impact:Strong
Examples
- •
75+ conditions treated
- •
~55k employees
- •
250+ active external innovation partners
- Proof Type:
News & Achievements
Impact:Strong
Examples
AbbVie to Acquire Gilgamesh Pharmaceuticals...
AbbVie Announces Positive Topline Results from Second Phase 3 UP-AA Trial...
Trust Indicators
- •
Prominently displayed news of acquisitions and positive clinical trial results.
- •
Detailed ESG (Environmental, Social and Governance) section with specific metrics (32% reduction in GHG emissions).
- •
Dedicated Investor Resources section with direct links to earnings reports and financial data.
- •
Clear articulation of a structured partnership process with named leaders.
- •
Links to company policies like 'Code of Conduct' and 'Pricing and Access'.
Scarcity Urgency Tactics
No itemsCalls To Action
Primary Ctas
- Text:
Learn more
Location:Homepage (Hero, Patient, Science, ESG sections)
Clarity:Somewhat Clear
- Text:
Browse jobs
Location:Homepage (Culture/Careers section)
Clarity:Clear
- Text:
Submit an opportunity
Location:Partner with Us page
Clarity:Clear
- Text:
Contact us
Location:Partner with Us page
Clarity:Clear
- Text:
LATEST EARNINGS RELEASE
Location:Homepage (Investor Resources section)
Clarity:Clear
The CTAs are generally effective for their specific audiences. High-intent users (investors, potential partners, job seekers) have very clear, direct CTAs. The general 'Learn more' CTA is ubiquitous and could be more specific and action-oriented (e.g., 'Explore Our Science', 'See Patient Stories') to better guide user journeys and improve information scent.
Messaging Gaps Analysis
Critical Gaps
There is no clear, dedicated pathway for Healthcare Professionals (HCPs) from the homepage. While they are a critical audience, the messaging lumps them in with the broader 'science' and 'patient' narratives without providing a specific information hub.
The 'Patients' section of the site is linked, but the homepage messaging for patients is more inspirational than practical. There is a lack of direct messaging around patient support programs or disease education resources on the main page.
Contradiction Points
No itemsUnderdeveloped Areas
The link to 'Pricing and Access of Our Innovative Medicines' is buried in the ESG section's quick links. Given the high public interest and scrutiny of pharmaceutical pricing, this topic could be addressed more proactively and transparently in the main narrative to build trust, even if it's a difficult subject.
While patient stories are used, they could be more deeply integrated. The story of 'Joe' is powerful but feels somewhat isolated. A more robust narrative library could strengthen the emotional connection.
Messaging Quality
Strengths
- •
Excellent audience segmentation and message tailoring, particularly for potential partners.
- •
A strong, consistent brand voice that balances scientific authority with a human-centric, purpose-driven tone.
- •
Effective use of data points and news updates as trust signals and social proof.
- •
The messaging successfully builds a corporate brand identity that transcends individual products, focusing on the company's culture, mission, and impact.
Weaknesses
- •
Over-reliance on the generic 'Learn More' CTA, which weakens user guidance.
- •
The homepage lacks a clear, dedicated entry point for the crucial Healthcare Professional (HCP) audience.
- •
The primary homepage message, 'We find answers that make life better...', while positive, is somewhat generic for the pharmaceutical industry.
Opportunities
- •
Create a dedicated 'For Healthcare Professionals' portal accessible from the homepage, featuring clinical data, medical information, and resources.
- •
Develop more specific, benefit-oriented CTAs to guide different user personas more effectively through the site.
- •
Feature a more prominent section on patient support and education to make the 'patient-centric' message more tangible and actionable.
- •
Integrate the ESG and corporate responsibility narrative more directly into the core brand story, rather than treating it as a separate section.
Optimization Roadmap
Priority Improvements
- Area:
Audience Segmentation (HCPs)
Recommendation:Add a primary navigation item or a prominent homepage block titled 'For Healthcare Professionals' leading to a dedicated content hub with clinical resources, pipeline information, and medical education.
Expected Impact:High
- Area:
Calls-to-Action
Recommendation:Replace generic 'Learn more' CTAs with more descriptive, action-oriented text. For example, change the CTA in the Science section to 'Explore Our Pipeline' or 'See Our R&D Approach'.
Expected Impact:Medium
- Area:
Patient-Centric Messaging
Recommendation:Elevate the visibility of patient support programs. Add a homepage module that directly links to 'Patient Support Resources' or 'Disease Information' to translate the aspirational patient message into practical help.
Expected Impact:High
Quick Wins
- •
Revise all 'Learn more' CTAs to be more specific to their content sections.
- •
Add a 'Healthcare Professionals' link to the main navigation or footer for improved access.
- •
Make the patient story of 'Joe' a rotating feature with other stories to show a broader commitment.
Long Term Recommendations
- •
Develop a more integrated content strategy that weaves patient stories, scientific innovation, and employee culture into a cohesive narrative across the entire site, rather than treating them as distinct content blocks.
- •
Create a more transparent and accessible messaging strategy around drug value, pricing, and access to proactively address public concerns and build deeper trust.
- •
Invest in creating richer content hubs for key therapeutic areas that serve multiple audiences (patients, HCPs, investors) with tailored information.
AbbVie's corporate website employs a sophisticated and highly effective messaging strategy designed to address a complex set of audiences, including patients, investors, potential employees, and strategic partners. The brand's core identity is built on the dual pillars of relentless scientific innovation and a deep, humanistic commitment to patient impact. The messaging architecture is clear and consistent, with a voice that is authoritative and purpose-driven without feeling cold or impersonal.
The website's greatest strength lies in its ability to tailor messages with precision. The 'Partner with Us' page is a masterclass in B2B communication, providing the exact level of detail and structural clarity that a potential scientific partner needs to see. Similarly, the investor and careers sections are direct and effective. The primary area for improvement is in serving its other key audiences—patients and healthcare professionals (HCPs). While the brand's inspirational, patient-centric tone is well-established, the messaging lacks clear, practical pathways for these groups on the homepage. There is a significant opportunity to make the patient focus more tangible with direct links to support programs and to create a dedicated information hub to better serve the needs of HCPs. Overall, AbbVie's messaging successfully positions it as a compassionate, innovative leader in the biopharmaceutical industry, but could enhance its effectiveness by creating more direct and utilitarian user journeys for its non-corporate audiences.
Growth Readiness
Growth Foundation
Product Market Fit
Strong
Evidence
- •
Successfully managing the transition away from reliance on blockbuster drug Humira, which faced U.S. patent expiration in 2023.
- •
Skyrizi and Rinvoq have become the new primary growth drivers, with combined sales expected to exceed $25 billion in 2025 and $31 billion by 2027, surpassing Humira's peak sales.
- •
Strong market uptake and share gains for Skyrizi and Rinvoq across multiple approved indications, particularly in immunology for conditions like IBD, psoriasis, and arthritis.
- •
Diversified portfolio across high-growth therapeutic areas including immunology, oncology, neuroscience, and aesthetics through strategic acquisitions like Allergan, ImmunoGen, and Cerevel Therapeutics.
- •
Robust R&D pipeline with approximately 90 compounds in development, and around 50 programs in mid and late-stage development, signaling future growth potential.
Improvement Areas
- •
Accelerate integration of recent large-scale acquisitions (ImmunoGen, Cerevel, Capstan) to realize synergies and bring acquired pipeline assets to market efficiently.
- •
Continue to expand indications for key growth drivers Skyrizi and Rinvoq to maximize their market potential and solidify their position as market leaders.
- •
Address performance challenges in the aesthetics business, which has faced headwinds and revised growth expectations downward.
Market Dynamics
The global pharmaceutical market is projected to grow at a CAGR of 5.2% to 7.1% from 2025 to 2031/2033.
Mature
Market Trends
- Trend:
AI and Machine Learning in Drug Discovery
Business Impact:AI is revolutionizing R&D by accelerating timelines, reducing costs, and increasing precision in identifying drug candidates and optimizing clinical trials. AbbVie's partnerships with tech firms like Xilio and Neomorph are crucial to leveraging this trend.
- Trend:
Rise of Biologics, Cell & Gene Therapies
Business Impact:Shift towards more complex and targeted therapies like biologics, ADCs, and CAR-T represents a major growth vector. AbbVie's acquisitions of ImmunoGen (ADCs) and Capstan Therapeutics (CAR-T) directly align with this trend.
- Trend:
Personalized Medicine and Real-World Evidence (RWE)
Business Impact:Increasing demand for treatments tailored to specific patient profiles requires investment in genomics, biomarker identification, and data analytics to prove value and secure reimbursement.
- Trend:
Pricing Pressure and Regulatory Scrutiny
Business Impact:Government policies like the U.S. Inflation Reduction Act and Medicare drug price negotiations pose a significant risk to revenue and profitability, requiring sophisticated market access strategies.
Excellent. AbbVie is executing a well-timed strategic pivot, replacing declining revenue from a legacy blockbuster with a new wave of high-growth products and a pipeline strengthened by acquisitions just as the market shifts towards advanced biologics and targeted therapies.
Business Model Scalability
High
High fixed costs in R&D and manufacturing, but low variable costs per unit sold, creating significant operating leverage once a drug is approved and commercialized.
High. Global sales infrastructure and established manufacturing capabilities allow for efficient scaling of new products worldwide. Recent investments in U.S. manufacturing further enhance this.
Scalability Constraints
- •
Complex manufacturing processes for biologics and cell therapies can be a bottleneck.
- •
Navigating disparate and lengthy regulatory approval processes in different international markets.
- •
High cost and complexity of integrating large-scale acquisitions into the existing corporate structure.
Team Readiness
Strong and proven. The leadership team has successfully navigated one of the largest patent cliffs in pharmaceutical history, demonstrating strategic foresight and execution capability.
Well-suited for growth. AbbVie operates with dedicated teams for Search & Evaluation, Business Development & Acquisitions, Global Alliances, and a venture arm, enabling a multi-pronged approach to sourcing external innovation. This structure is core to their growth strategy.
Key Capability Gaps
Deep expertise in nascent but high-potential areas like in-vivo CAR-T and RNA-based therapies, which they are actively acquiring (e.g., Capstan Therapeutics).
Enhanced capabilities in digital health and data science to fully leverage real-world evidence and personalized medicine trends.
Growth Engine
Acquisition Channels
- Channel:
Strategic M&A and Licensing
Effectiveness:High
Optimization Potential:Medium
Recommendation:Continue disciplined M&A focused on late-stage assets and novel technology platforms in core therapeutic areas (oncology, neuroscience, immunology) to de-risk the pipeline and enter new high-growth sub-markets.
- Channel:
Internal R&D Pipeline Development
Effectiveness:High
Optimization Potential:High
Recommendation:Prioritize and accelerate late-stage pipeline assets with the highest commercial potential, such as those in oncology (Teliso-V, Elahere) and neuroscience (Tavapadon), to deliver the next wave of growth.
- Channel:
Commercial Sales Force & Medical Affairs (Physician Engagement)
Effectiveness:High
Optimization Potential:Medium
Recommendation:Focus commercial efforts on maximizing market share for Skyrizi and Rinvoq, particularly in competitive indications like inflammatory bowel disease, by emphasizing clinical differentiation and securing favorable formulary access.
Customer Journey
The 'customer' journey involves multiple stakeholders (patient, physician, payer). Conversion begins with clinical data influencing physician prescription, followed by securing payer reimbursement, and ensuring patient adherence through support programs.
Friction Points
- •
Payer restrictions and prior authorization hurdles delaying patient access to therapy.
- •
Competition from biosimilars and other branded products in crowded therapeutic areas.
- •
Complexity of patient support programs for specialty drugs.
Journey Enhancement Priorities
{'area': 'Market Access & Reimbursement', 'recommendation': 'Develop compelling health economics and outcomes research (HEOR) data to demonstrate superior value to payers, thereby easing access restrictions for key products like Skyrizi and Rinvoq.'}
{'area': 'Physician Education', 'recommendation': "Leverage digital channels and real-world evidence to provide ongoing education to healthcare providers on the clinical benefits and optimal use of AbbVie's newer, more complex therapies."}
Retention Mechanisms
- Mechanism:
Label Expansion (New Indications)
Effectiveness:High
Improvement Opportunity:Systematically pursue and secure regulatory approvals for Skyrizi and Rinvoq in new immunology indications (e.g., alopecia areata, vitiligo) to expand the addressable patient population and extend product lifecycles.
- Mechanism:
Patient Support Programs
Effectiveness:Medium
Improvement Opportunity:Enhance patient support services with digital tools to improve medication adherence and patient engagement, creating stickiness for long-term therapies.
- Mechanism:
Demonstrating Superior Clinical Outcomes
Effectiveness:High
Improvement Opportunity:Conduct head-to-head clinical trials against key competitors to generate data that solidifies market-leading positions and defends against new entrants.
Revenue Economics
Extremely favorable for successful drugs. The pharmaceutical model involves massive upfront R&D investment (high effective 'customer acquisition cost') offset by high-margin revenue over a long patent-protected period (very high 'lifetime value').
Not directly applicable in the traditional sense, but the portfolio's return on R&D investment is the key metric. The success of Skyrizi and Rinvoq demonstrates a highly effective model for replacing revenue post-patent cliff.
High. The company is successfully managing the Humira revenue decline, with total company revenue returning to growth in 2025, just two years after the U.S. loss of exclusivity.
Optimization Recommendations
- •
Optimize R&D spend by using AI/ML to improve candidate selection and trial design, increasing the probability of success and reducing development timelines.
- •
Continue to aggressively defend intellectual property for key products to maximize the duration of market exclusivity.
- •
Implement efficient manufacturing and supply chain processes to protect gross margins, especially for complex biologics.
Scale Barriers
Technical Limitations
- Limitation:
Pipeline Concentration Risk
Impact:High
Solution Approach:Continued diversification through strategic M&A and partnerships to reduce dependency on a small number of blockbuster drugs and therapeutic areas.
- Limitation:
Clinical Trial Failures
Impact:High
Solution Approach:Focus on assets with strong proof-of-concept data and validated mechanisms of action. Utilize advanced analytics and adaptive trial designs to increase the likelihood of success in late-stage trials.
Operational Bottlenecks
- Bottleneck:
Post-Merger Integration Complexity
Growth Impact:Integrating large companies like Allergan, ImmunoGen, and Cerevel introduces significant operational, cultural, and technical challenges that can distract from core growth activities.
Resolution Strategy:Establish dedicated integration management offices (IMOs) with clear mandates, timelines, and metrics for success to ensure synergies are realized without disrupting ongoing business momentum.
- Bottleneck:
Biologics Manufacturing Scale-Up
Growth Impact:The complexity of manufacturing advanced biologics, ADCs, and cell therapies can constrain supply and delay product launches.
Resolution Strategy:Proactive investment in expanding manufacturing capacity, as seen with the $195 million Illinois plant expansion, and partnering with contract manufacturing organizations (CMOs) for specialized capabilities.
Market Penetration Challenges
- Challenge:
Humira Biosimilar Erosion
Severity:Critical
Mitigation Strategy:This is being actively and successfully managed by driving rapid uptake of Skyrizi and Rinvoq to offset the decline, a strategy that has become a textbook case for navigating a patent cliff.
- Challenge:
Intense Competition in Key Therapeutic Areas
Severity:Major
Mitigation Strategy:Compete on clinical differentiation by generating superior data, pursuing new indications where AbbVie can be first-in-class or best-in-class, and employing aggressive commercial strategies to win formulary access. Key competitors include J&J, Pfizer, Roche, Merck, and Eli Lilly.
- Challenge:
Payer Pushback and Pricing Pressure
Severity:Major
Mitigation Strategy:Generate robust real-world evidence and health economic data to demonstrate the long-term value and cost-effectiveness of innovative medicines to justify premium pricing.
Resource Limitations
Talent Gaps
- •
Top-tier scientists and researchers in emerging fields like gene editing, RNA therapeutics, and AI-driven drug discovery.
- •
Data scientists and bioinformaticians to translate large-scale biological data into actionable insights.
- •
Regulatory affairs specialists with experience in cell and gene therapies.
Significant but manageable. AbbVie maintains strong cash flow but carries substantial debt from acquisitions ($70B). Continued disciplined capital allocation is required to fund R&D, further M&A, and shareholder returns (dividends).
Infrastructure Needs
Expansion of specialized manufacturing facilities for next-generation therapies (e.g., cell therapies, ADCs).
Investment in a robust data infrastructure to support AI/ML initiatives and the analysis of large-scale clinical and real-world data.
Growth Opportunities
Market Expansion
- Expansion Vector:
Geographic Expansion in Emerging Markets
Potential Impact:Medium
Implementation Complexity:High
Recommended Approach:Pursue regulatory approvals and build commercial infrastructure in high-growth regions like Asia-Pacific, tailoring market access strategies to local reimbursement systems.
- Expansion Vector:
Indication Expansion
Potential Impact:High
Implementation Complexity:Medium
Recommended Approach:Systematically execute the clinical development plan to secure approvals for Skyrizi and Rinvoq in all addressable immunology indications, maximizing the lifecycle value of these cornerstone assets.
Product Opportunities
- Opportunity:
Advance the Late-Stage Oncology Pipeline
Market Demand Evidence:High unmet need in solid tumors (NSCLC, CRC) and hematologic malignancies.
Strategic Fit:Strengthens the oncology franchise, a key pillar of AbbVie's diversified growth strategy.
Development Recommendation:Prioritize registrational trials for promising assets like Teliso-V (c-Met ADC) and leverage the newly acquired Elahere (FRα ADC) to build a leading antibody-drug conjugate portfolio.
- Opportunity:
Capitalize on the Neuroscience Pipeline
Market Demand Evidence:Growing prevalence of neurodegenerative and psychiatric disorders (Parkinson's, Alzheimer's, MDD).
Strategic Fit:Neuroscience is AbbVie's fastest-growing sector and a key area for diversification and long-term growth.
Development Recommendation:Aggressively advance the pipeline acquired with Cerevel and the newly acquired Bretisilocin for MDD to establish market leadership in psychiatry and neurology.
- Opportunity:
Enter the High-Growth Obesity Market
Market Demand Evidence:Massive and rapidly growing global market with significant unmet need.
Strategic Fit:Represents a major new therapeutic area for diversification.
Development Recommendation:Leverage the partnership with Gubra to develop a differentiated amylin analog (GUB014295) that can compete in a crowded but lucrative market.
Channel Diversification
- Channel:
Digital Health & Therapeutics
Fit Assessment:High
Implementation Strategy:Partner with or acquire digital health companies to develop 'beyond the pill' solutions that improve patient outcomes and create data-driven insights, enhancing the value proposition for patients and payers.
- Channel:
AI-Driven Drug Discovery Platforms
Fit Assessment:High
Implementation Strategy:Expand partnerships with AI-native biotechnology companies (similar to the Neomorph deal) to accelerate the identification of novel targets and drug candidates, improving R&D productivity.
Strategic Partnerships
- Partnership Type:
Biotech Licensing & Co-Development
Potential Partners
Early-stage biotechs with novel platforms (e.g., gene editing, radiopharmaceuticals)
Academic research institutions
Expected Benefits:Access to cutting-edge science and external innovation to supplement the internal pipeline, as clearly outlined on their 'Partner with Us' page. De-risks R&D by sharing costs and gaining external validation.
- Partnership Type:
Technology & Data Partnerships
Potential Partners
AI/ML companies specializing in life sciences
Real-world data providers (e.g., Flatiron, Tempus)
Expected Benefits:Enhance R&D efficiency, optimize clinical trial design, and generate compelling evidence for market access and reimbursement.
Growth Strategy
North Star Metric
Ex-Humira Revenue Growth Rate
This is the single most important metric demonstrating the company's successful transformation and ability to grow beyond its legacy blockbuster. It directly measures the performance of the new growth platform (Skyrizi, Rinvoq, Vraylar, acquisitions, etc.) and is the primary focus for investors and leadership.
Achieve and sustain a high single-digit compound annual revenue growth rate (ex-Humira) through 2029, consistent with company guidance.
Growth Model
Diversified R&D and Strategic M&A-Led Growth
Key Drivers
- •
Successful commercial execution for Skyrizi & Rinvoq.
- •
Positive late-stage clinical trial readouts.
- •
Strategic, value-accretive acquisitions of new technologies and pipeline assets.
- •
Effective post-merger integration to realize synergies.
A dual-engine approach: 1) Maximize the commercial potential of the current on-market growth portfolio. 2) Aggressively reinvest cash flow into both internal R&D and external M&A/licensing to build the next wave of innovative products.
Prioritized Initiatives
- Initiative:
Maximize Immunology Leadership with Skyrizi & Rinvoq
Expected Impact:High
Implementation Effort:Medium
Timeframe:Ongoing - 2027
First Steps:Secure regulatory approvals for all planned indications and aggressively pursue favorable formulary placement against competitors.
- Initiative:
Integrate ImmunoGen and Cerevel to Build Oncology & Neuroscience Franchises
Expected Impact:High
Implementation Effort:High
Timeframe:12-24 Months
First Steps:Establish joint integration teams, align R&D priorities, and harmonize commercial strategies for acquired and existing assets.
- Initiative:
Advance Late-Stage Pipeline Assets to Regulatory Submission
Expected Impact:High
Implementation Effort:High
Timeframe:24-48 Months
First Steps:Fully fund and staff pivotal trials for key assets like tavapadon (Parkinson's) and Teliso-V (NSCLC) to ensure timely data readouts and submissions.
Experimentation Plan
High Leverage Tests
{'area': 'Novel Therapeutic Modalities', 'experiment': 'Make strategic venture investments or small-scale acquisitions in next-generation platforms like radiopharmaceuticals or in-vivo gene editing to gain early access and expertise.'}
{'area': 'New Commercial Models', 'experiment': 'Pilot value-based pricing agreements with a major payer for a key drug, linking reimbursement to patient outcomes to demonstrate confidence and secure favorable access.'}
For R&D 'experiments', success is measured by progression through clinical phases and eventual probability of technical and regulatory success (PTRS). For commercial experiments, success is measured by market share gains, formulary win rates, and net price realization.
Continuous evaluation of external innovation opportunities through the dedicated Business Development and AbbVie Ventures teams. Formal portfolio review on a quarterly and annual basis.
Growth Team
Maintain and enhance the existing highly effective, decentralized structure comprising distinct but collaborative units: 1) Business Development & Acquisitions, 2) Search & Evaluation, 3) AbbVie Ventures, 4) Global Alliances, and 5) Internal R&D Leadership. Ensure a strong central strategy function aligns priorities across these groups.
Key Roles
- •
Chief Strategy Officer
- •
Head of Business Development
- •
Therapeutic Area Heads (for R&D and Commercial)
- •
Head of Integration Management
Actively recruit top scientific and business talent from biotech hubs. Foster an internal culture that embraces calculated risk-taking in R&D and M&A. Provide ongoing training in new scientific fields and data analytics to upskill the existing workforce.
AbbVie is in a position of remarkable strength, having successfully executed one of the most challenging strategic pivots in the pharmaceutical industry: navigating the patent cliff of its mega-blockbuster, Humira. The company's growth foundation is solid, anchored by the exceptional performance of its next-generation immunology drugs, Skyrizi and Rinvoq, which are not just replacing but are on track to significantly exceed Humira's peak revenues.
The company's growth engine is a powerful, dual-pronged machine combining potent internal R&D with an aggressive and disciplined M&A strategy. This has allowed AbbVie to diversify into high-growth areas like oncology and neuroscience, effectively de-risking its future and building a formidable pipeline. Recent acquisitions of ImmunoGen, Cerevel, and Capstan Therapeutics are testaments to this strategy, positioning AbbVie at the forefront of trends like antibody-drug conjugates and advanced CNS therapies.
The primary barrier to growth remains the immense pressure of the pharmaceutical market itself: intense competition from other global giants, persistent pricing pressure from payers, and the inherent risk of clinical trial failures. However, AbbVie has proven its ability to manage these challenges, particularly the competitive threat, through superior commercial execution and clinical differentiation.
Key growth opportunities lie in maximizing the lifecycle of Skyrizi and Rinvoq through label expansions, advancing the high-potential late-stage assets in oncology and neuroscience, and making a strategic entry into the obesity market. Strategic partnerships and leveraging AI in drug discovery will be critical enablers to enhance R&D productivity.
Our recommendation is to adopt 'Ex-Humira Revenue Growth Rate' as the North Star Metric to maintain focus on the new, diversified business. The strategic priorities are clear: 1) Dominate the immunology market with the current portfolio; 2) Successfully integrate recent acquisitions to build out the oncology and neuroscience pillars; and 3) Accelerate the most promising late-stage pipeline assets to market. By continuing to execute this strategy, AbbVie is well-positioned not just to survive the post-Humira era, but to emerge as a more resilient, diversified, and powerful leader in the biopharmaceutical industry.
Legal Compliance
AbbVie demonstrates a mature and comprehensive approach to privacy. The website provides a clear, accessible 'Privacy Notice' in the footer, which is layered and detailed. It effectively outlines the categories of personal data collected (from consumers, HCPs, and job applicants), the purposes for processing, and data sharing practices. Crucially, AbbVie offers a separate 'Consumer Health Data Privacy Notice,' indicating a proactive stance towards specific and sensitive data types governed by emerging laws like Washington's My Health My Data Act. The policies address global requirements, with links to country-specific notices, acknowledging its international operations. The framework covers data collected both online and offline from various sources, including third parties, which is a critical disclosure for a company engaged in extensive research and patient programs.
The 'Terms of Use' are readily accessible and written with a satisfactory level of clarity for a legal document. The terms effectively establish the rules of engagement for website users, including prohibitions on disrupting electronic information or circumventing security. Key strengths include robust disclaimers of warranties, stating that information is provided 'AS IS' and that AbbVie does not warrant the accuracy or suitability of the content. The terms also clearly delineate AbbVie's intellectual property rights regarding its trademarks and content, prohibiting unauthorized use. A critical and well-executed component is the disclaimer for third-party content and links, absolving AbbVie of responsibility for information on external sites, which is vital for managing regulatory risk when linking to other resources.
The website employs a modern cookie consent management approach, evidenced by the 'Cookie Settings' link in the footer. This functionality suggests that users are provided with granular control over different categories of cookies (e.g., functional, advertising), which is a key requirement under GDPR and other modern privacy laws. While the initial banner experience isn't captured in the provided text, the existence of a settings center implies a mechanism for obtaining user consent before deploying non-essential cookies. The privacy policy also discloses the use of cookies and similar technologies for purposes like tailored advertising and analytics, which is a necessary transparency measure.
AbbVie's data protection framework appears robust and aligned with major global regulations like GDPR and CCPA/CPRA. The privacy notice details data subject rights and the presence of a 'Your Privacy Choices' portal is a best-in-class feature for centralizing and simplifying the exercise of these rights (e.g., access, deletion, opt-out). This indicates a mature process for handling Data Subject Access Requests (DSARs). For its global operations, AbbVie acknowledges the need for international data transfer mechanisms and provides various country-specific privacy notices, demonstrating a commitment to localized compliance. The detailed explanation of data collection from healthcare professionals and patient program participants highlights an understanding of the specific data protection needs of different stakeholder groups.
AbbVie demonstrates a clear commitment to digital accessibility by providing a dedicated 'Accessibility Statement' link in the website footer. This is a crucial first step in meeting legal requirements under the Americans with Disabilities Act (ADA) and adhering to global standards like the Web Content Accessibility Guidelines (WCAG). Recent U.S. Health and Human Services (HHS) rules mandate that healthcare organizations receiving federal funds must meet WCAG 2.1 Level AA standards, making this a significant compliance area. While the statement itself requires review to confirm the specific conformance target (e.g., WCAG 2.1 AA), its prominent placement signifies that accessibility is a recognized compliance priority.
As a pharmaceutical company, AbbVie is subject to intense industry-specific regulation, primarily from the U.S. Food and Drug Administration (FDA) regarding the marketing and promotion of prescription drugs. The website's structure smartly mitigates risk by focusing on corporate communications, investor relations, disease awareness, and scientific partnerships, rather than direct-to-consumer product advertising. A key compliance strength is the use of an exit-site modal ('You are about to leave the AbbVie website') when linking to product-specific sites. This is a critical tool for ensuring that information is accessed only by the intended audience (e.g., residents of a specific country) and for providing legally required 'fair balance' of risk and benefit information. The website's content also includes necessary 'Forward-Looking Statements' disclaimers in its investor relations materials, which is required by securities law.
Compliance Gaps
Potential Vendor Risk: A recent data breach was filed by AbbVie, potentially stemming from a third-party vendor (Lash Group/Cencora). While AbbVie's direct compliance may be strong, this highlights the critical, and often challenging, area of third-party vendor risk management and supply chain security.
Practical Accessibility Implementation: While an accessibility statement is present, ongoing and dynamic content updates can introduce accessibility issues. Without a hands-on audit, it is impossible to verify if the site consistently meets the WCAG 2.1 AA standards it is likely subject to.
Compliance Strengths
- •
Mature Privacy Framework: The use of layered privacy notices, a dedicated Consumer Health Data notice, a 'Your Privacy Choices' portal, and country-specific policies demonstrates a best-practice approach to global data protection.
- •
Robust Legal Disclaimers: The Terms of Use contain clear and comprehensive disclaimers regarding medical advice, information accuracy, and third-party content, effectively managing legal risk.
- •
Strategic Content Segregation: The website architecture and use of exit-site pop-ups effectively segregate heavily regulated product information from general corporate communications, which is a cornerstone of FDA compliance for pharmaceutical web properties.
- •
Explicit Commitment to Accessibility: The presence of a formal 'Accessibility Statement' indicates a high level of awareness and commitment to meeting legal obligations for users with disabilities.
- •
Comprehensive Corporate Ethics Program: AbbVie's stated Ethics and Compliance Program, which includes adherence to the Code of Business Conduct and various industry codes, provides a strong foundation for its legal and regulatory posture.
Risk Assessment
- Risk Area:
Regulatory Enforcement (FDA/Off-Label Promotion)
Severity:High
Recommendation:Continue the current successful strategy of strictly separating corporate/disease-awareness content from audience-gated, country-specific product information. Ensure all patient stories and marketing materials are rigorously reviewed for compliance with 'fair balance' and on-label promotion requirements.
- Risk Area:
Third-Party Data Breach
Severity:High
Recommendation:In light of the recent vendor-related incident, conduct a thorough review and audit of the security and data protection clauses and practices of all third-party vendors who handle personal or health-related data. Enhance vendor due diligence and monitoring protocols.
- Risk Area:
Evolving Global Privacy Laws
Severity:Medium
Recommendation:Maintain the current proactive stance by continuously monitoring new and amended data privacy laws globally. Regularly update the Privacy Notices and the 'Your Privacy Choices' portal to ensure ongoing compliance with new requirements and data subject rights.
- Risk Area:
Accessibility Litigation
Severity:Medium
Recommendation:Commission regular, independent audits of the website against WCAG 2.1 AA (or higher) standards to validate the claims in the Accessibility Statement. Implement a process for ongoing accessibility testing, especially after significant website updates or redesigns.
High Priority Recommendations
- •
Enhance Third-Party Vendor Risk Management: Following the recent data breach notification, immediately prioritize a comprehensive audit of data-handling vendors to mitigate supply chain risk, which represents a significant legal and reputational vulnerability.
- •
Conduct and Document Regular Accessibility Audits: Move beyond the policy statement by commissioning and documenting regular third-party audits against WCAG 2.1 AA standards to create a defensible record of compliance and ensure the site is genuinely accessible to all users, mitigating legal risk under the ADA and HHS regulations.
- •
Maintain Proactive Monitoring of Global Regulations: Continue to invest in legal and compliance resources to monitor the rapidly changing landscape of both data privacy (new state laws) and pharmaceutical marketing regulations to maintain the company's strong compliance posture.
Overall, AbbVie's website (abbvie.com) demonstrates a highly sophisticated and mature legal compliance framework that functions as a strategic business asset. The company's legal positioning is exceptionally strong, reflecting the high-stakes regulatory environment of the global pharmaceutical industry. The clear segregation of corporate and product information, robust use of disclaimers, and a comprehensive, multi-layered privacy infrastructure are standout strengths. This meticulous approach not only mitigates significant legal risks from regulators like the FDA and data protection authorities but also builds trust with critical stakeholders—patients, healthcare professionals, and investors. The proactive adoption of a 'Consumer Health Data Privacy Notice' and a 'Your Privacy Choices' portal positions AbbVie as a leader in adapting to the complex web of modern data privacy laws. While the core on-site compliance is excellent, the primary area of risk appears to be in the operational execution of its policies, specifically regarding third-party vendor oversight and the continuous, practical implementation of digital accessibility standards.
Visual
Design System
Corporate Professional
Excellent
Advanced
User Experience
Navigation
Horizontal Top Bar with 'More' Dropdown
Intuitive
Excellent
Information Architecture
Logical
Clear
Light
Conversion Elements
- Element:
CTA Button ('Explore careers')
Prominence:Medium
Effectiveness:Effective
Improvement:Increase visual weight or use a more contrasting color to draw more attention for talent acquisition goals.
- Element:
CTA Button ('Partner with us')
Prominence:High
Effectiveness:Effective
Improvement:The button is well-placed and clear. Consider adding a short, compelling value proposition nearby to increase motivation to click.
- Element:
Accordion/Expandable Sections ('Areas of Interest')
Prominence:Medium
Effectiveness:Effective
Improvement:This is an excellent way to manage dense information. Ensure the most critical partnership areas are listed first or visually prioritized.
- Element:
Link ('Submit an opportunity')
Prominence:Medium
Effectiveness:Somewhat effective
Improvement:The inline text link is subtle. For a key action like submitting a partnership opportunity, elevating this to a button-style CTA would significantly improve its visibility and actionability.
Assessment
Strengths
- Aspect:
Clear Brand Storytelling
Impact:High
Description:The website effectively communicates AbbVie's mission to discover and deliver innovative medicines. The use of patient-centric imagery and headlines like 'Patients are at the heart of everything we do' powerfully conveys the company's purpose and values. This aligns with their stated brand campaigns which focus on the people behind the medicines.
- Aspect:
Professional & Trustworthy Aesthetic
Impact:High
Description:The clean layout, high-quality photography, and consistent use of a blue and purple color palette create a sense of trust, scientific expertise, and professionalism. This is crucial for a biopharmaceutical company whose audiences include healthcare professionals, investors, and patients making critical health decisions.
- Aspect:
Scalable Information Architecture
Impact:Medium
Description:The use of clear top-level navigation ('Who We Are', 'Science', 'Patients') and expandable accordion sections on deeper pages allows for the presentation of complex information without overwhelming the user. This structure effectively serves diverse audiences, from potential employees to research partners.
- Aspect:
Consistent Visual Identity
Impact:High
Description:There is a strong, consistent application of brand elements—logo, typography, color, and imagery style—across all pages shown. This creates a unified and memorable brand experience, reinforcing AbbVie's corporate identity.
Weaknesses
- Aspect:
Subtle Primary CTAs
Impact:Medium
Description:While aesthetically pleasing, some key call-to-action buttons, like 'Explore careers', use a 'ghost button' style (outline only). This reduces their visual prominence compared to the surrounding content, potentially lowering engagement for key conversion goals like talent acquisition.
- Aspect:
Generic Stock-like Photography
Impact:Low
Description:Some of the imagery, particularly in the corporate and science sections, has the feel of high-quality stock photography. While professional, it can lack the authenticity that custom, narrative-driven photography could provide to further enhance the 'patient-at-the-heart' story.
- Aspect:
Heavy Reliance on Blue
Impact:Low
Description:The extensive use of blue, while conveying trust and stability, can feel monotonous in large blocks. Introducing a broader, but still controlled, secondary color palette for interactive elements or specific content modules could add more visual interest and better guide the user's eye.
Priority Recommendations
- Recommendation:
Elevate Key 'Partnership' and 'Career' CTAs
Effort Level:Low
Impact Potential:High
Rationale:For a company that relies on strategic partnerships and attracting top talent, making these conversion points as prominent as possible is critical. Changing the 'Explore careers' ghost button to a solid fill and transforming the 'Submit an opportunity' link into a button will significantly increase visibility and user action, directly supporting key business objectives.
- Recommendation:
Diversify Hero Section Content Strategy
Effort Level:Medium
Impact Potential:Medium
Rationale:The main hero banner is visually appealing but static. Implementing a rotating hero that showcases different key messages targeting various audiences (e.g., one for investors focusing on financial results, one for patients on support programs, one for scientists on R&D breakthroughs) could make the homepage more dynamic and immediately relevant to a wider range of visitors.
- Recommendation:
Introduce Micro-interactions and Animations
Effort Level:Medium
Impact Potential:Low
Rationale:The site feels very static. Adding subtle micro-interactions, such as hover effects on cards, animated counters for statistics (like the '250+ partners' section), or smooth-scrolling transitions, would enhance the user experience, making it feel more modern, polished, and engaging without distracting from the core content.
Mobile Responsiveness
Excellent
The design appears to use a fluid grid system that adapts cleanly. Content stacks logically in a single column on mobile, with font sizes and spacing adjusted appropriately for readability on smaller screens.
Mobile Specific Issues
No itemsDesktop Specific Issues
No itemsThis visual audit of AbbVie.com reveals a mature, professional, and highly effective corporate website that successfully communicates the company's brand identity as a leading biopharmaceutical firm. AbbVie's mission—to discover and deliver innovative medicines—is clearly articulated through a patient-centric narrative and a trustworthy design aesthetic.
1. Design System Coherence and Brand Identity Expression:
The website employs an advanced and coherent design system. The brand identity is expressed with excellence through the consistent use of its corporate color palette (primarily blues and purples), clean typography, and high-quality, professional imagery. The overall design style is 'Corporate Professional,' which is appropriate for its industry and diverse target audience, including healthcare professionals, patients, investors, and potential partners. This consistency builds brand recognition and reinforces a message of stability and scientific rigor.
2. Visual Hierarchy and Information Architecture:
The visual hierarchy is effective, guiding users logically through the content. Headlines are clearly distinguished from body copy, and key sections are visually separated by color blocks and ample white space. The information architecture is logical, with top-level navigation that clearly segments content for different audiences ('Science', 'Patients', 'Sustainability'). Deeper pages, like the 'Partner with Us' section, use accordions to manage detailed information effectively, reducing cognitive load and allowing users to explore areas of interest without being overwhelmed.
3. Navigation Patterns and User Flow Optimization:
The primary navigation is a standard horizontal top bar, which is intuitive for users. On mobile, it condenses into a clear icon-based system. User flows appear to be well-considered; for example, a user interested in partnerships can easily navigate from the 'Science' section to a dedicated page outlining opportunities and areas of interest. The journey is clear and unobstructed.
4. Mobile Responsiveness and Cross-Device Experience:
Based on the structure of the provided screenshots, the website demonstrates a strong, responsive design. The single-column layout in the long scroll indicates a mobile-first or adaptive approach where content reflows logically. Elements are well-spaced, and typography likely scales appropriately, ensuring a positive user experience across devices.
5. Visual Conversion Elements and Call-to-Action (CTA) Effectiveness:
CTAs are present for key business goals like partnerships and careers. However, their visual treatment could be optimized. The 'Partner with Us' CTA is prominent, but others, like 'Explore careers' and particularly the text-link 'Submit an opportunity', lack the visual weight needed to maximize clicks. Adopting a consistent, high-contrast style for all primary CTAs would improve conversion rates for these important user actions.
6. Visual Storytelling and Content Presentation:
The website excels at visual storytelling, especially in its patient-focused sections. The "I AM..." campaign is a powerful example, using authentic-feeling portraits to connect the company's scientific work to real-life impact. The combination of human-centric imagery with data points and scientific content creates a balanced narrative that appeals to both emotion and logic, effectively communicating the value and purpose of AbbVie's work.
Discoverability
Market Visibility Assessment
AbbVie's digital presence firmly establishes it as a global leader in the biopharmaceutical industry. The corporate website functions as a high-authority hub, communicating scientific leadership and a patient-centric mission. Content focuses on innovation, R&D breakthroughs, and corporate strength, effectively positioning AbbVie as a top-tier entity for investors, potential partners, and top talent. Recent omnichannel campaigns like 'I Am' further bolster this by humanizing the brand, showcasing patient stories alongside the scientists behind the medicines to build broader public trust and brand affinity.
Digitally, AbbVie commands high visibility for its key products (e.g., Skyrizi, Rinvoq, Botox) and for branded corporate searches. However, its primary challenge is managing the narrative around the decline of its former blockbuster, Humira, due to biosimilar competition. Competitors like Johnson & Johnson, Pfizer, Merck, and Eli Lilly fiercely contest visibility in core therapeutic areas such as immunology and oncology. While AbbVie's revenue ranks it among the top global pharma companies, its digital market share for non-branded, disease-state searches is an area for strategic growth, as these are often captured by more specialized medical sites or competitors with broader patient education platforms.
The website demonstrates very high potential for engaging its key stakeholder audiences. For investors, the site provides clear pathways to financial results, reports, and presentations. For potential partners, the 'Partner with Us' section is a sophisticated B2B tool detailing specific R&D interests and providing direct contact channels, crucial for pipeline growth. For talent acquisition, it showcases a strong corporate culture and career opportunities. For patients and Healthcare Professionals (HCPs), the corporate site serves as a primary trust-building and directional hub, guiding them toward specific product and medical information websites, which is standard and appropriate for the regulated pharma industry.
AbbVie's digital strategy shows a strong understanding of global markets. The main .com website serves as a global portal, but it effectively uses prompts and clear navigation to direct users to country-specific sites. This is critical for regulatory compliance, as information on pharmaceutical products must be tailored to the approvals and regulations of each specific region. This global-local structure allows AbbVie to maintain a consistent corporate brand while adapting its product and medical information to diverse geographic markets.
AbbVie's digital content provides excellent coverage of its core strategic therapeutic areas: immunology, oncology, neuroscience, and eye care. The 'Science' and 'Partner with Us' sections are particularly robust, detailing specific areas of interest and technological platforms (e.g., ADCs, genetic medicine). This demonstrates deep expertise and communicates its R&D focus to the scientific and business communities. The newsroom consistently features positive clinical trial results and strategic acquisitions, reinforcing AbbVie's position at the forefront of innovation in these fields.
Strategic Content Positioning
Content is strategically aligned with the distinct journeys of its core audiences. For investors and partners (a B2B journey), the content provides deep, rational information on financials, pipeline, and partnership criteria to facilitate decision-making. For prospective employees, it highlights culture and impact. For patients and the general public, the content focuses on building emotional connection and trust through patient stories and high-level brand mission statements, effectively positioning AbbVie as a patient-first organization at the top of the funnel before directing them to more specific resources.
While AbbVie effectively disseminates news about clinical trial results, there is a significant opportunity to translate this data into broader thought leadership. This could involve creating more accessible content—such as expert interviews, forward-looking articles on disease treatment, and roundtable discussions with their R&D leaders—to frame the implications of their science. This would capture a wider audience and solidify their position not just as a drug manufacturer, but as a visionary leader shaping the future of medicine.
A key competitive gap is the lack of comprehensive, unbranded patient education hubs on AbbVie's main corporate site. Competitors like Eli Lilly have launched direct-to-consumer platforms (LillyDirect) that offer an end-to-end patient experience, including educational resources and telehealth access. Developing robust, non-promotional content around the diseases AbbVie treats could capture significant search traffic, build early-stage patient trust, and provide a valuable resource for HCPs to share with their patients, creating a competitive advantage in market education.
Brand messaging is exceptionally consistent across the digital presence. The core tenets of being a science-driven, patient-centric organization dedicated to solving tough health challenges are reinforced on the homepage, in patient stories, within the R&D sections, and in sustainability reports. This disciplined messaging creates a powerful and coherent brand identity that strengthens trust and recognition among all stakeholder groups.
Digital Market Strategy
Market Expansion Opportunities
- •
Develop comprehensive, unbranded 'Therapeutic Area Hubs' for key disease states like Crohn's disease, rheumatoid arthritis, and psoriasis. These hubs would serve as authoritative educational resources for patients and HCPs, capturing significant organic search traffic and establishing AbbVie as the go-to source for information.
- •
Expand digital engagement with the scientific community by creating dedicated content platforms for researchers, featuring detailed pipeline updates, scientific publications, and webinars with AbbVie's R&D leaders.
- •
Explore digital health solutions and 'beyond the pill' services, such as patient support apps or partnerships with digital health platforms, to add value and build deeper patient relationships in a manner similar to competitors' initiatives.
Stakeholder Engagement Optimization
- •
Enhance the 'Partner with Us' portal with case studies of successful collaborations and a more streamlined submission process to increase the conversion rate of high-quality partnership opportunities.
- •
Create a more personalized digital experience for returning HCPs, allowing them to easily access medical information, clinical trial data, and resources relevant to their specialty.
- •
Proactively manage investor perceptions by creating a dedicated digital narrative around the 'post-Humira' growth story, showcasing the combined strength of Skyrizi, Rinvoq, and the broader pipeline through interactive data visualizations and executive commentary.
Brand Authority Initiatives
- •
Launch an annual 'AbbVie State of Innovation' digital report or interactive experience to showcase R&D progress, future therapeutic landscapes, and key scientific achievements, positioning the company as a forward-thinking industry leader.
- •
Elevate the public profiles of key scientists and executives as thought leaders through featured articles, speaking engagements at major non-pharma conferences (e.g., tech, business), and active, professional social media engagement.
- •
Invest in creating high-production value documentary-style content that follows the journey of a medicine from discovery in the lab to its impact on a patient's life, humanizing the brand and demonstrating its societal contribution.
Competitive Positioning Improvements
- •
Directly counter the Humira biosimilar narrative by amplifying the success and expanding indications of Skyrizi and Rinvoq, positioning them as the new standard of care in immunology.
- •
Leverage the acquisition of Allergan by creating a more integrated digital narrative that showcases the synergistic strength of its combined portfolio in aesthetics and neuroscience.
- •
Benchmark and aim to outperform competitors in digital engagement with HCPs by adopting more innovative, data-driven, and personalized communication strategies, as demonstrated by competitors like Eli Lilly.
Business Impact Assessment
Market share will be indirectly measured through digital signals such as branded search volume for key growth products (Skyrizi, Rinvoq) versus competitor products. A primary indicator is the rate of decline in search interest for Humira versus the rate of growth for its successors, reflecting the success of the digital transition strategy.
For R&D partnerships, the key metric is the number of qualified collaboration opportunities submitted through the 'Partner with Us' portal. For talent, success is measured by the volume and quality of applicants sourced through the careers section for critical R&D and commercial roles. For HCPs, metrics include referral traffic to medical information portals and engagement with clinical trial content.
Brand authority can be measured by share of voice in online media for key therapeutic areas, the volume of unsolicited positive media mentions, and backlinks from high-authority medical, academic, and financial domains. Rankings in industry reports on innovation and corporate reputation are also key benchmarks.
Benchmarking will involve comparing AbbVie's digital presence against key competitors (Pfizer, Merck, Eli Lilly, J&J) on several fronts: organic visibility for strategic, non-branded keywords; engagement rates on professional social networks like LinkedIn; and the sophistication of their patient and HCP digital resources. A key benchmark is the effectiveness of their digital narrative in shaping investor confidence compared to peers.
Strategic Recommendations
High Impact Initiatives
- Initiative:
Develop 'Therapeutic Area Authority Hubs'
Business Impact:High
Market Opportunity:Capture a significant share of the non-branded search market for diseases AbbVie treats, building brand trust and preference with patients and HCPs long before a treatment decision is made. This addresses a competitive gap where rivals are more active in patient education.
Success Metrics
- •
Organic traffic growth for disease-related keywords
- •
Engagement metrics (time on page, downloads)
- •
Growth in qualified leads to patient support programs
- Initiative:
Launch 'The AbbVie Innovation Engine' Digital Experience
Business Impact:High
Market Opportunity:Proactively shape the market narrative around AbbVie's future beyond Humira. This initiative directly addresses investor concerns and attracts top scientific talent and partners by showcasing the depth and dynamism of the R&D pipeline.
Success Metrics
- •
Investor sentiment analysis
- •
Media mentions focusing on the pipeline
- •
Volume and quality of inbound partnership inquiries
- •
Traffic and engagement on the digital experience itself
- Initiative:
Create a Personalized HCP Engagement Portal
Business Impact:Medium
Market Opportunity:Differentiate AbbVie from competitors by providing a superior, data-driven digital experience for healthcare professionals. This can increase loyalty and prescribing confidence by making it easier for HCPs to find the precise clinical information they need.
Success Metrics
- •
HCP registration and return visit rates
- •
Time-to-information for key clinical data
- •
Positive feedback from HCPs via surveys
- •
Increased engagement with digital medical education materials
The overarching strategy is to digitally position AbbVie as the architect of the future of medicine in its core therapeutic areas. This requires shifting the public and investor narrative from a company defined by a single past blockbuster (Humira) to a diversified, forward-looking innovator driven by a powerful R&D engine (Skyrizi, Rinvoq, and a deep pipeline). Every digital touchpoint should reinforce this transition, showcasing scientific prowess, future growth drivers, and a profound, lasting impact on patients' lives.
Competitive Advantage Opportunities
- •
Achieve digital dominance in immunology by leveraging the market-leading positions of Skyrizi and Rinvoq to create the most comprehensive and authoritative online resource for patients and HCPs in this space.
- •
Utilize the highly specific and well-articulated 'Partner with Us' section as a strategic weapon to attract the most promising external biotech innovations, outpacing competitors in building the next-generation pipeline.
- •
Build a deeper, more trusted relationship with patients through authentic storytelling and valuable, unbranded educational content, creating a brand moat based on trust that competitors focused solely on product marketing cannot easily replicate.
AbbVie's digital market presence is that of a mature, top-tier pharmaceutical leader. Its corporate website, Abbvie.com, excels as a multi-stakeholder communications hub, effectively serving the distinct needs of investors, potential partners, and prospective employees. The messaging is consistent, authoritative, and reinforces the company's core identity as a science-driven, patient-centric organization.
The primary strategic challenge AbbVie faces is navigating the post-Humira era. While the company has been successful financially in transitioning to new growth drivers like Skyrizi and Rinvoq , its corporate digital narrative must accelerate this story to shape market perception proactively. The current digital presence is strong on corporate communications but has a clear opportunity to build deeper engagement with patients and Healthcare Professionals (HCPs) through market education.
Compared to competitors like Eli Lilly, which is aggressively pursuing direct-to-consumer digital health platforms , AbbVie's approach is more traditional. The key strategic recommendation is to augment its strong corporate presence by developing unbranded 'Therapeutic Area Authority Hubs'. These content platforms would serve as definitive educational resources for key diseases, capturing vast early-stage search interest from patients and HCPs. This initiative would build a powerful brand halo, establish trust, and create a significant competitive advantage by owning the educational journey.
Furthermore, launching a dynamic digital experience focused on 'The AbbVie Innovation Engine' would directly address the need to reframe the company's narrative around its diverse and promising pipeline. This would serve to reassure investors, attract the best scientific talent, and solidify its position as a preferred destination for biotech partnerships. By executing on these content-driven strategies, AbbVie can leverage its digital presence not just to communicate its current success, but to define its future leadership in the pharmaceutical industry.
Strategic Priorities
Strategic Priorities
- Title:
Accelerate Pipeline Diversification via Disciplined, High-Impact M&A
Business Rationale:While the Skyrizi/Rinvoq platform is successfully replacing Humira revenue, long-term growth and risk mitigation depend on reducing dependency on the immunology franchise. Strategic acquisitions in high-growth areas like oncology, neuroscience, and potentially obesity are critical to build new revenue pillars and access novel technology platforms (e.g., ADCs, CAR-T).
Strategic Impact:Transforms AbbVie from an immunology-centric company into a truly diversified biopharmaceutical leader with multiple, durable growth engines, making it more resilient to competition and patent cycles in any single therapeutic area.
Success Metrics
- •
Value of M&A deals closed in strategic growth areas ($B)
- •
Number of late-stage (Phase II/III) assets added to the pipeline
- •
Projected peak sales of acquired assets vs. acquisition cost
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Market Position
- Title:
Establish Digital Ecosystems to Lead in Patient & HCP Engagement
Business Rationale:Competitors are moving 'beyond the pill' with integrated digital health platforms. AbbVie has a competitive gap in unbranded patient education and dedicated HCP resources. Building authoritative 'Therapeutic Area Hubs' will capture audiences early, build trust, and create a powerful competitive moat based on value-added services, not just the drug itself.
Strategic Impact:Shifts the business model from purely selling therapeutics to creating holistic disease management ecosystems. This deepens relationships with both patients and physicians, improves adherence, and generates valuable real-world data, creating a sustainable advantage.
Success Metrics
- •
Growth in organic traffic to unbranded educational content
- •
HCP registration and engagement rates on a dedicated professional portal
- •
Patient enrollment in digital support programs
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Customer Strategy
- Title:
Systematize Post-Merger Integration to Maximize Acquisition ROI
Business Rationale:AbbVie's growth strategy is heavily dependent on large-scale acquisitions (Allergan, ImmunoGen, Cerevel). The analysis identifies integration complexity as a key operational bottleneck. Developing a dedicated, repeatable playbook for rapidly integrating R&D, commercial operations, and company cultures is essential to unlock synergies and accelerate time-to-market for acquired assets.
Strategic Impact:Creates a core competency in M&A integration, turning it from a high-risk operational challenge into a predictable, value-creation engine. This allows AbbVie to acquire and absorb new companies more efficiently and effectively than competitors.
Success Metrics
- •
Reduction in time to achieve projected cost/revenue synergies by X%
- •
Key talent retention rate from acquired companies
- •
Time-to-market for key pipeline assets from acquired entities
Priority Level:HIGH
Timeline:Quick Win (0-3 months)
Category:Operations
- Title:
Pioneer Value-Based Commercial Models to Counter Pricing Pressure
Business Rationale:Global pricing pressure and regulatory scrutiny (e.g., Inflation Reduction Act) are the most significant long-term threats to profitability. Proactively developing and piloting value-based contracts—where reimbursement is tied to clinical outcomes—demonstrates confidence in product efficacy and aligns AbbVie with the future of healthcare reimbursement.
Strategic Impact:Positions AbbVie as an innovative partner to payers, rather than an adversary. This strategy defends premium pricing for breakthrough therapies, improves market access, and creates a new competitive dimension based on proven real-world value.
Success Metrics
- •
Number of pilot value-based agreements signed with major payers
- •
Net price realization for products under value-based contracts
- •
Improved formulary access and reduced restrictions for key drugs
Priority Level:MEDIUM
Timeline:Long-term Vision (12+ months)
Category:Revenue Model
- Title:
Launch a Proactive 'Innovation Engine' Narrative to Reshape Market Perception
Business Rationale:The market narrative still carries the shadow of the Humira patent cliff. It is crucial to proactively shift investor and public perception to focus on AbbVie's future: a diversified innovator with a deep pipeline. A concerted brand initiative showcasing the strength of the R&D engine and recent acquisitions will solidify the company's position as a forward-looking leader.
Strategic Impact:Moves the corporate brand identity beyond its legacy blockbuster, bolstering investor confidence, improving stock valuation, and attracting top-tier scientific talent and partners who want to join a company defined by its future, not its past.
Success Metrics
- •
Increase in positive analyst ratings citing pipeline strength
- •
Share of voice in media focused on 'innovation' vs. 'Humira decline'
- •
Inbound interest from high-quality biotech partnership targets
Priority Level:HIGH
Timeline:Quick Win (0-3 months)
Category:Brand Strategy
AbbVie's immediate imperative is to cement its successful pivot from a single-blockbuster dependency by flawlessly integrating recent acquisitions to build dominant oncology and neuroscience franchises. Concurrently, it must evolve its engagement model by creating value-added digital ecosystems for patients and HCPs, securing its future as a resilient and diversified biopharmaceutical leader.
The ability to execute massive strategic pivots, combining a world-class commercialization engine with a disciplined M&A strategy to successfully replace legacy revenue and build new, market-leading therapeutic franchises.
A dual-engine growth model that maximizes the commercial dominance of its current on-market portfolio (Skyrizi/Rinvoq) while aggressively reinvesting cash flow into acquiring and developing a deep pipeline of next-generation assets in adjacent high-growth therapeutic areas.