eScore
criver.comThe eScore is a comprehensive evaluation of a business's online presence and effectiveness. It analyzes multiple factors including digital presence, brand communication, conversion optimization, and competitive advantage.
Charles River demonstrates exceptional digital presence through high-authority content like the 'Eureka' blog, which firmly establishes them as a scientific thought leader. Their content strategy is well-aligned with the complex B2B user journey, targeting different stages from initial research to direct sales inquiry. The website reflects a strong global presence, catering to key markets in North America, Europe, and Asia, although voice search optimization is an underdeveloped area common in this B2B sector.
Content Authority: The creation of in-depth, PhD-level scientific content establishes immense credibility and authority, attracting a highly specialized and valuable audience.
Develop a formal strategy for voice search and conversational queries, focusing on answering specific scientific and logistical questions that researchers might ask, to capture emerging search behaviors.
The brand communication is world-class, anchored by the incredibly powerful and specific social proof of having worked on '80% of FDA-approved drugs'. This single data point validates all other claims of expertise and reliability. Messaging is effectively tailored to different audiences, from C-suite executives (risk reduction, speed-to-market) to R&D scientists (technical expertise, specific methodologies), creating a compelling narrative of a proven, indispensable partner.
Competitive Messaging: The '80% of FDA-approved drugs' statistic is a masterstroke of differentiation, creating a unique and quantifiable claim of market leadership that is difficult for any competitor to counter.
Incorporate more direct customer voice and testimonials into the main service pages to further humanize the partnership narrative and provide immediate social proof at the point of consideration.
The website presents a professional and credible user experience, but it suffers from conversion friction due to inconsistent call-to-action (CTA) design and information-dense layouts. While the navigation is logical, the lack of a clear visual hierarchy for CTAs can confuse users and dilute the intended conversion paths. The cross-device experience is functional, but text-heavy sections can be fatiguing on mobile, increasing cognitive load for users trying to scan for information.
Clear Information Architecture: The top-level navigation and site structure are logical, allowing users to effectively self-segment and find relevant categories for their needs.
Establish and consistently apply a hierarchical CTA system (e.g., Primary: solid button, Secondary: ghost button, Tertiary: link with icon) across the entire site to guide users more effectively towards key conversion actions like 'Consult an Expert'.
Credibility is Charles River's greatest strength, built on a foundation of decades of experience and a strong regulatory track record. The company's comprehensive legal and compliance framework, especially concerning GDPR and industry-specific regulations like GLP, is a major asset that builds immense trust. Third-party validation is powerfully demonstrated by the '80% of FDA drugs' claim and customer case studies, while an extensive library of scientific content serves as profound evidence of expertise.
Third-party Validation & Customer Success Evidence: The statistic of contributing to 80% of FDA-approved drugs is the ultimate form of customer success evidence, providing unparalleled credibility and risk mitigation for potential clients.
Increase transparency in the 'Terms & Conditions' by updating the document to reflect the latest data privacy regulations (e.g., CPRA) and adding a formal, public Accessibility Statement.
Charles River has a deep, sustainable competitive moat built on three pillars: its dominant and hard-to-replicate research models business, a fully integrated end-to-end portfolio, and a stellar brand reputation built over decades. These advantages create extremely high switching costs for clients who rely on their integrated services for mission-critical drug development programs. While they are a leader in an established market, their significant investments in high-growth areas like cell & gene therapy demonstrate strong innovation indicators.
Moat Sustainability: The combination of a fully integrated portfolio, a dominant position in research models, and a trusted regulatory track record creates a highly defensible market position that is exceptionally difficult and expensive for competitors to replicate.
Develop and market a proprietary, data-driven service layer leveraging the vast amount of historical preclinical data to offer predictive insights, creating a new, highly defensible competitive advantage.
As a mature market leader, Charles River has a proven model for expansion, with a strong global footprint and clear signals of targeting high-growth markets like APAC. The business enjoys favorable unit economics with a very high customer lifetime value, though scaling is capital-intensive due to high fixed costs for facilities and specialized talent. The primary constraints on scalability are the operational complexity and the competitive market for acquiring highly specialized scientific personnel.
Market Expansion Signals: The company has a clear strategy for growth by targeting emerging biotech hubs and investing heavily in the rapidly expanding cell and gene therapy manufacturing sector.
Develop a more scalable service model, such as the proposed 'CRL Catalyst' platform, to more efficiently capture the long tail of innovative but smaller biotech firms without relying solely on the high-touch enterprise sales model.
The business model is exceptionally coherent and aligned with the major secular trend of R&D outsourcing in the biopharmaceutical industry. Revenue streams are well-diversified across discovery, safety, and manufacturing, creating a resilient and deeply integrated value proposition. The company demonstrates a clear strategic focus on expanding into high-margin, high-growth areas like biologics manufacturing, indicating efficient resource allocation and strong market timing.
Revenue Model Optimization: The three core business segments (RMS, DSA, Manufacturing) are highly synergistic, allowing for effective cross-selling and the creation of a sticky, end-to-end client journey that maximizes lifetime value.
Innovate the pricing model for early-stage biotech partners by exploring risk-sharing or milestone-based contracts to better align with their funding cycles and capture value from their potential success.
Charles River wields significant market power, evidenced by its position as a market leader and its ability to command premium prices based on a reputation for quality and reliability. The company's influence is substantial, as it plays a role in the development of the vast majority of approved drugs, effectively setting industry standards for preclinical research. This market leadership provides considerable leverage with partners and a strong defense against competitive pressures.
Market Influence & Pricing Power: The company's indispensable role in the biopharma ecosystem and its unparalleled track record give it significant pricing power and the ability to shape industry standards and best practices.
Systematically leverage its vast proprietary data to publish annual industry trend reports, further solidifying its market influence and creating a powerful asset for digital PR and lead generation.
Business Overview
Business Classification
B2B Services & Products
Contract Research Organization (CRO) / Contract Development & Manufacturing Organization (CDMO)
Life Sciences
Sub Verticals
- •
Drug Discovery & Development
- •
Preclinical Safety & Efficacy Testing
- •
Biologics & Pharmaceutical Manufacturing
- •
Cell & Gene Therapy Solutions
- •
Research Models & Services
Mature
Maturity Indicators
- •
Founded in 1947 and publicly traded (NYSE: CRL).
- •
Global operations in over 20 countries with more than 130 facilities.
- •
Comprehensive, end-to-end service portfolio covering the entire drug development lifecycle.
- •
Long-standing reputation, having worked on over 80% of FDA-approved drugs in the last five years.
- •
History of strategic acquisitions to expand capabilities.
Enterprise
Steady
Revenue Model
Primary Revenue Streams
- Stream Name:
Discovery and Safety Assessment (DSA)
Description:Provides outsourced services for drug discovery, safety and efficacy testing, and bioanalytical services. This is the largest segment, acting as a critical partner for clients' early-stage R&D pipelines.
Estimated Importance:Primary
Customer Segment:Pharmaceutical and Biotechnology Companies
Estimated Margin:High
- Stream Name:
Research Models and Services (RMS)
Description:Production and sale of genetically standardized laboratory research models (mice, rats) and related services. This is the foundational business of the company.
Estimated Importance:Secondary
Customer Segment:Academic Institutions, Government Agencies, Pharma/Biotech
Estimated Margin:Medium
- Stream Name:
Manufacturing Solutions
Description:Provides biologics testing, microbial solutions, and specialized CDMO services for cell and gene therapies. This is a high-growth area focused on the manufacturing and quality control phases.
Estimated Importance:Secondary
Customer Segment:Pharmaceutical and Biotechnology Companies
Estimated Margin:High
Recurring Revenue Components
- •
Long-term strategic partnership agreements
- •
Multi-year service contracts for drug development programs
- •
Recurring sales of research models to established labs
- •
Ongoing quality control and manufacturing support contracts
Pricing Strategy
Contract-Based & Fee-for-Service
Premium
Opaque
Pricing Psychology
- •
Value-Based Pricing
- •
Expertise & Reputation Pricing
- •
Bundled Service Offerings
Monetization Assessment
Strengths
- •
Diversified revenue across three major segments of the biopharma value chain.
- •
Deeply embedded in client R&D and manufacturing workflows, leading to high switching costs.
- •
Premium pricing power based on reputation, quality, and regulatory expertise.
- •
Balanced client portfolio including large pharma, biotech, and academic institutions.
Weaknesses
- •
High sensitivity to fluctuations in the R&D spending of pharmaceutical and biotech companies.
- •
Revenue can be impacted by funding cycles in the biotech sector.
- •
High fixed costs associated with global laboratory and manufacturing facilities.
Opportunities
- •
Rapid growth in biologics, cell, and gene therapies presents a significant expansion opportunity for the Manufacturing Solutions segment.
- •
Increasing trend of outsourcing in the pharmaceutical industry to reduce costs and accelerate timelines.
- •
Strategic acquisitions to enter new high-growth therapeutic areas or technological capabilities.
Threats
- •
Intense competition from other large CROs/CDMOs like LabCorp, IQVIA, and WuXi AppTec.
- •
Regulatory shifts, particularly concerning animal welfare, could impact the RMS segment.
- •
Economic downturns leading to reduced R&D funding and project cancellations.
Market Positioning
End-to-End Scientific Partner
Market Leader
Target Segments
- Segment Name:
Global Pharmaceutical Companies
Description:Large, multinational pharmaceutical corporations with extensive drug pipelines and significant R&D budgets.
Demographic Factors
- •
Global presence
- •
Large employee base (>10,000)
- •
Annual revenue in the multi-billions
Psychographic Factors
- •
Focus on risk mitigation and regulatory compliance
- •
Value reliability, scalability, and established reputation
- •
Seek long-term strategic partnerships
Behavioral Factors
- •
Outsource non-core R&D and manufacturing activities
- •
Engage in multi-year, multi-service contracts
- •
High volume of testing and research model needs
Pain Points
- •
Pressure to reduce internal fixed costs
- •
Need to accelerate drug development timelines
- •
Managing complex global supply chains for research and manufacturing
Fit Assessment:Excellent
Segment Potential:Medium
- Segment Name:
Biotechnology Companies
Description:Small, mid-size, and large biotech firms, often focused on novel therapeutic modalities like cell & gene therapy. Can range from venture-backed startups to established public companies.
Demographic Factors
Varying sizes, from small teams to thousands of employees
Often pre-revenue or with limited commercial products
Psychographic Factors
- •
Highly focused on innovation and speed to clinic
- •
Value scientific expertise and flexibility
- •
Often operate with a 'virtual' or lean model
Behavioral Factors
Heavy reliance on outsourcing for most or all development and manufacturing
Funding is often tied to clinical milestones
Pain Points
- •
Lack of internal infrastructure and expertise
- •
Need for rapid, efficient progress to secure next funding round
- •
Navigating complex regulatory pathways for novel therapies
Fit Assessment:Excellent
Segment Potential:High
- Segment Name:
Academic & Government Institutions
Description:Universities, research hospitals, and government agencies (e.g., NIH) conducting basic and translational research.
Demographic Factors
Non-profit or publicly funded
Focus on scientific discovery and publication
Psychographic Factors
Prioritize quality and reproducibility of research models
Budget-conscious
Behavioral Factors
Purchase research models and specific testing services
Less likely to engage in large, integrated drug development programs
Pain Points
Need for high-quality, specific research models for grant-funded research
Limited internal capacity for specialized assays or animal care
Fit Assessment:Good
Segment Potential:Low
Market Differentiation
- Factor:
Comprehensive Service Portfolio
Strength:Strong
Sustainability:Sustainable
- Factor:
Global Scale and Infrastructure
Strength:Strong
Sustainability:Sustainable
- Factor:
Regulatory Expertise & Reputation
Strength:Strong
Sustainability:Sustainable
- Factor:
Proprietary Research Models
Strength:Moderate
Sustainability:Sustainable
Value Proposition
To be the indispensable global partner for accelerating the entire drug development journey, from basic research to commercial manufacturing, by providing an unparalleled portfolio of essential products, services, and scientific expertise.
Excellent
Key Benefits
- Benefit:
Accelerated Time-to-Market
Importance:Critical
Differentiation:Somewhat unique
Proof Elements
End-to-end service integration reduces handoffs and delays.
Claim of working on >80% of FDA-approved drugs in the past 5 years.
- Benefit:
Reduced R&D Risk
Importance:Critical
Differentiation:Somewhat unique
Proof Elements
Decades of regulatory experience and scientific expertise.
High-quality, standardized models and testing procedures.
- Benefit:
Access to Specialized Expertise and Technology
Importance:Important
Differentiation:Unique
Proof Elements
Deep expertise in high-growth areas like cell & gene therapy.
Global network of specialized facilities and scientists.
- Benefit:
Increased Operational & Capital Efficiency
Importance:Important
Differentiation:Common
Proof Elements
Clients can avoid building and maintaining expensive in-house infrastructure.
Flexible outsourcing model allows clients to scale R&D efforts up or down.
Unique Selling Points
- Usp:
The industry's most comprehensive, fully integrated portfolio spanning the entire drug discovery and development continuum from a single provider.
Sustainability:Long-term
Defensibility:Strong
- Usp:
Unmatched global scale and decades of experience, serving as a foundational partner to the biopharmaceutical industry.
Sustainability:Long-term
Defensibility:Strong
Customer Problems Solved
- Problem:
The immense cost, complexity, and time required to bring a new therapy to market.
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Lack of in-house specialized infrastructure and scientific expertise, especially for smaller biotech firms.
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Navigating the complex and evolving global regulatory landscape for drug approval.
Severity:Major
Solution Effectiveness:Partial
Value Alignment Assessment
High
The business model is perfectly aligned with the secular industry trend of increasing R&D outsourcing, driven by rising drug development complexity and cost pressures.
High
The value proposition directly addresses the primary pain points of both large pharma (cost efficiency, speed) and small biotech (access to infrastructure, expertise).
Strategic Assessment
Business Model Canvas
Key Partners
- •
Pharmaceutical Companies
- •
Biotechnology Companies
- •
Academic & Government Research Institutions
- •
Technology & Equipment Suppliers
- •
Logistics & Cold Chain Providers
Key Activities
- •
Contract Research (Discovery, Preclinical)
- •
Contract Manufacturing (Biologics, Cell & Gene Therapy)
- •
Breeding & Maintenance of Research Models
- •
Scientific & Regulatory Consulting
- •
Quality Control & Bioanalytical Testing
Key Resources
- •
Global network of GLP/GMP compliant facilities
- •
Highly skilled scientific and technical workforce
- •
Proprietary portfolio of research models
- •
Intellectual property and validated methodologies
- •
Strong client relationships and brand reputation
Cost Structure
- •
Personnel Costs (Specialized Scientists and Technicians)
- •
Facilities & Equipment (High Fixed Costs)
- •
Research & Development for new services
- •
Animal Care & Husbandry
- •
Regulatory Compliance & Quality Assurance
Swot Analysis
Strengths
- •
Dominant market position and strong brand recognition.
- •
Integrated, end-to-end service portfolio creates high customer stickiness.
- •
Global scale provides operational efficiencies and a wide client reach.
- •
Deep scientific and regulatory expertise acts as a significant competitive moat.
Weaknesses
- •
Vulnerability to biotech funding cycles and pharmaceutical R&D budget cuts.
- •
Negative public perception and ethical scrutiny related to animal testing.
- •
High fixed-cost structure can impact margins during downturns.
- •
Complex operations present integration challenges with acquisitions.
Opportunities
- •
Capitalize on the rapid growth of advanced modalities like cell & gene therapy and biologics.
- •
Expand CDMO capabilities to capture more of the manufacturing value chain.
- •
Leverage data assets and AI to create predictive discovery and safety services.
- •
Further penetration into emerging APAC life sciences markets.
Threats
- •
Intensifying competition from global and niche CRO/CDMO players.
- •
Development of New Alternative Methods (NAMs) that could reduce reliance on animal models.
- •
Increased price pressure from clients.
- •
Global geopolitical instability impacting supply chains and client operations.
Recommendations
Priority Improvements
- Area:
Technology & Data Strategy
Recommendation:Invest in and promote an integrated data platform for clients, offering real-time project tracking, data analysis, and predictive insights. This would further embed CRL in client workflows and create a new data-centric value proposition.
Expected Impact:High
- Area:
Market Perception
Recommendation:Proactively lead the narrative on ethical animal use and the development/adoption of New Alternative Methods (NAMs). Position the company as a leader in the '3Rs' (Replacement, Reduction, Refinement) to mitigate reputational risk.
Expected Impact:Medium
- Area:
Service Integration
Recommendation:Streamline the customer journey across the RMS, DSA, and Manufacturing segments to create a truly seamless 'concept-to-commercialization' experience, improving client retention and increasing the value of each partnership.
Expected Impact:Medium
Business Model Innovation
- •
Develop risk-sharing or milestone-based pricing models for partnerships with early-stage biotech companies, aligning CRL's success with its clients' clinical and commercial success.
- •
Launch a dedicated strategic consulting arm focused on R&D portfolio strategy and regulatory navigation, leveraging CRL's vast industry-wide experience.
- •
Create a 'virtual R&D' platform service that provides startups with a fully outsourced, integrated team and infrastructure to move from discovery to IND filing.
Revenue Diversification
- •
Expand further into post-approval services, such as long-term safety monitoring and batch release testing for commercial drugs.
- •
Monetize anonymized, aggregated preclinical data as a service to help clients with target validation and predictive toxicology.
- •
Build out educational and training services for the biopharmaceutical industry, leveraging internal expertise.
Charles River Laboratories (CRL) has an exceptionally strong and mature business model, positioning it as an indispensable partner in the global biopharmaceutical industry. Its primary strength lies in its comprehensive, end-to-end portfolio of services and products, which spans the entire drug development lifecycle. This integration creates significant competitive moats through high switching costs, deep client relationships, and economies of scale. The business is well-aligned with the persistent industry trend of R&D and manufacturing outsourcing. Future growth is strategically anchored in the high-margin, rapidly expanding fields of biologics and cell & gene therapies, for which CRL is aggressively building its CDMO capabilities. Key challenges revolve around navigating the cyclical nature of biotech funding, managing the ethical and regulatory complexities of its foundational animal model business, and fending off intense competition. Strategic evolution should focus on digital transformation to leverage its vast data assets, further integrating its service offerings for a seamless client experience, and innovating its partnership models to capture more value, particularly from the vibrant early-stage biotech ecosystem. The scalability of the model is proven, and its future success will depend on its ability to remain at the scientific and technological forefront of drug development.
Competitors
Competitive Landscape
Mature
Moderately concentrated, with the top tier of the market functioning as an oligopoly dominated by a few large players like Charles River, Labcorp, and IQVIA.
Barriers To Entry
- Barrier:
High Capital Investment
Impact:High
Description:Building and maintaining state-of-the-art, cGMP-compliant laboratories, manufacturing facilities, and vivariums requires enormous capital expenditure.
- Barrier:
Regulatory Compliance and Expertise
Impact:High
Description:Deep expertise in navigating complex global regulations (FDA, EMA, etc.) and a proven track record of successful submissions are essential. Achieving certifications like GLP (Good Laboratory Practice) is a significant hurdle.
- Barrier:
Scientific Talent and Specialized Expertise
Impact:High
Description:Access to a deep bench of experienced scientists, toxicologists, and manufacturing experts is critical and highly competitive.
- Barrier:
Reputation and Client Trust
Impact:High
Description:Biopharmaceutical companies entrust their most valuable assets (drug candidates) to CROs/CDMOs. A long-standing reputation for quality, reliability, and confidentiality is paramount and takes years to build.
- Barrier:
Economies of Scale
Impact:Medium
Description:Large, established players can offer integrated, end-to-end services at a cost and speed that new entrants cannot match.
Industry Trends
- Trend:
Growth of Biologics, Cell & Gene Therapies
Impact On Business:This is a primary growth driver. Charles River's focus on areas like CAR-T and viral vector manufacturing positions it well, but also intensifies competition with other specialists in this domain.
Timeline:Immediate
- Trend:
Integration of AI and Machine Learning in Drug Discovery
Impact On Business:AI platforms can accelerate early-stage discovery, potentially disrupting traditional preclinical testing models. This presents both a threat and an opportunity for partnership to validate AI-discovered candidates.
Timeline:Near-term
- Trend:
Focus on Personalized and Precision Medicine
Impact On Business:Drives demand for specialized research models (e.g., genetically engineered models), companion diagnostics, and smaller, more flexible manufacturing batches, all of which are CRL's strengths.
Timeline:Immediate
- Trend:
Increased Outsourcing by Pharma and Biotech
Impact On Business:The overall market is growing as companies of all sizes, from virtual biotechs to big pharma, continue to outsource R&D and manufacturing to improve efficiency and access specialized expertise.
Timeline:Immediate
- Trend:
Shift Towards Alternative Testing Models (NAMs)
Impact On Business:Growing ethical and scientific pressure to reduce animal testing creates demand for New Approach Methodologies (NAMs). While a threat to the traditional research models business, CRL's investment in these alternatives is a crucial adaptive strategy.
Timeline:Near-term
Direct Competitors
- →
Labcorp Drug Development (formerly Covance)
Market Share Estimate:High
Target Audience Overlap:High
Competitive Positioning:Positions as a global, end-to-end provider leveraging its vast diagnostic and clinical trial laboratory network to provide unique data-driven insights.
Strengths
- •
Unmatched scale in central laboratory services for clinical trials.
- •
Integration with a massive diagnostics business provides unique data insights (genomics, biomarkers).
- •
Strong focus on late-stage clinical development and toxicology.
- •
Global reach and significant capacity.
Weaknesses
- •
Less emphasis on the very early 'basic research' and discovery side compared to Charles River's research models.
- •
Brand perception can be more corporate and less 'scientific partner' oriented.
- •
Potential for integration challenges and bureaucracy due to immense scale.
Differentiators
Leveraging patient diagnostic data to inform clinical trial design and recruitment.
Global network of central labs is a key competitive moat for multi-regional clinical trials.
- →
IQVIA
Market Share Estimate:High
Target Audience Overlap:Medium
Competitive Positioning:Positions as a leader in human health data science and clinical research, combining data, technology, analytics, and deep domain expertise.
Strengths
- •
Dominant in clinical trial management and execution (Phase I-IV).
- •
Proprietary health data and analytics platforms (IQVIA CORE) are a major differentiator.
- •
Strong consulting and commercialization services for market access and post-launch activities.
- •
Technology-driven solutions for trial optimization.
Weaknesses
- •
Limited presence in early discovery, preclinical research, and manufacturing. Not an end-to-end player in the same way as CRL.
- •
Primarily focused on the 'clinical' part of R&D, not the 'research' part.
- •
Services can be perceived as more expensive due to the data and tech overlay.
Differentiators
The use of real-world evidence (RWE) and proprietary data sets to design and execute smarter, faster clinical trials.
Strong technology stack for managing clinical data and operations.
- →
Lonza Group
Market Share Estimate:Medium-High
Target Audience Overlap:Medium
Competitive Positioning:A premier, pure-play Contract Development and Manufacturing Organization (CDMO) focused on biologics and cell & gene therapy.
Strengths
- •
World-class expertise in biologics manufacturing, particularly mammalian cell culture and microbial fermentation.
- •
Leading CDMO for cell and gene therapy, including viral vector and plasmid DNA production (a direct competitor to CRL's manufacturing arm).
- •
Strong reputation for quality and technical expertise in complex molecule manufacturing.
- •
Global network of large-scale and flexible manufacturing facilities.
Weaknesses
- •
Not a full-service CRO; lacks the discovery and preclinical testing services that CRL offers.
- •
More focused on the 'D' and 'M' of CDMO, rather than the 'R' of CRO.
- •
Higher cost structure associated with premium manufacturing services.
Differentiators
Deep, specialized focus on the manufacturing of complex biological drugs.
Proprietary expression systems and manufacturing platforms (e.g., GS Xceed®).
- →
Thermo Fisher Scientific (PPD & Patheon)
Market Share Estimate:High
Target Audience Overlap:High
Competitive Positioning:The ultimate 'one-stop-shop' for science, combining a leading CRO (PPD) and CDMO (Patheon) with an unparalleled catalog of lab equipment, reagents, and consumables.
Strengths
- •
Incredible synergy potential: can supply a client from the earliest research reagents all the way through commercial manufacturing and clinical trial logistics.
- •
PPD provides a top-tier global clinical research offering.
- •
Patheon provides a comprehensive CDMO service for small molecules and biologics.
- •
Massive global commercial and distribution footprint.
Weaknesses
- •
The sheer scale can make the client experience feel fragmented across different divisions (Reagents vs. Services vs. Equipment).
- •
Potential for internal channel conflict.
- •
Integration of large acquisitions (like PPD) takes time and can lead to cultural and operational friction.
Differentiators
The ability to create highly integrated, bundled offerings that span products and services, which is unique in the market.
Deep relationships with virtually every lab and pharma company in the world through their core products business.
Indirect Competitors
- Name:
Niche CROs/CDMOs (e.g., WuXi AppTec, Catalent)
Description:Companies that offer deep expertise in specific areas. Catalent is a leader in drug delivery technologies and dose form manufacturing. WuXi AppTec offers a highly integrated platform with a strong presence in China.
Threat Level:High
Potential For Direct Competition:They are already direct competitors in many segments, but clients may choose them for specific expertise, potentially breaking up an end-to-end service package that CRL would prefer to offer.
- Name:
Academic and Institutional Core Facilities
Description:Universities and research institutes that offer specialized services (e.g., animal modeling, genomic sequencing) to smaller biotechs and researchers, often at a lower cost.
Threat Level:Low
Potential For Direct Competition:Unlikely to compete at a commercial scale, but they can capture very early-stage R&D work from academic spin-outs and startups.
- Name:
AI-Powered Drug Discovery Companies (e.g., Recursion, Schrödinger)
Description:Technology companies that use AI/ML to identify and design novel drug candidates, bypassing traditional discovery methods. They partner with pharma, but also develop their own pipelines.
Threat Level:Medium
Potential For Direct Competition:Their threat is disruptive. They don't compete on services, but they could devalue the traditional discovery process where CRL has a strong foothold. They are also potential high-value clients for preclinical validation services.
Competitive Advantage Analysis
Sustainable Advantages
- Advantage:
Dominance in Research Models
Sustainability Assessment:Highly sustainable, though facing long-term pressure from alternative testing methods.
Competitor Replication Difficulty:Hard
Description:CRL's history and unparalleled portfolio of research models, particularly genetically engineered models (GEMS), creates a deep competitive moat. The infrastructure, expertise, and decades of data are very difficult to replicate.
- Advantage:
Fully Integrated End-to-End Portfolio
Sustainability Assessment:Highly sustainable.
Competitor Replication Difficulty:Hard
Description:The ability to support a client from basic research and discovery through manufacturing is a powerful value proposition, reducing hand-offs, accelerating timelines, and de-risking development. Only a few competitors can truly match this breadth.
- Advantage:
Strong Regulatory Track Record and Brand Reputation
Sustainability Assessment:Highly sustainable.
Competitor Replication Difficulty:Hard
Description:The statistic that 80% of FDA-approved drugs were worked on by CRL is a powerful marketing tool and testament to their embedded role in the industry. This trust is built over decades and countless successful programs.
Temporary Advantages
- Advantage:
Lead in a Specific New Technology or Service
Estimated Duration:1-3 years
Description:Expertise in a newly emerging area, like a specific type of cell therapy manufacturing, can provide a temporary edge until competitors build or acquire similar capabilities.
Disadvantages
- Disadvantage:
Premium Price Perception
Impact:Major
Addressability:Moderately
Description:As a market leader with a comprehensive offering, CRL may be perceived as more expensive than smaller, niche providers, potentially losing cost-sensitive clients for point solutions.
- Disadvantage:
Operational Complexity
Impact:Major
Addressability:Difficult
Description:Managing a vast and diverse portfolio of services globally is inherently complex. Ensuring a consistently high-quality and seamless client experience across all business units is a constant challenge.
- Disadvantage:
Ethical and Social Scrutiny of Animal Research
Impact:Minor
Addressability:Moderately
Description:The foundational research models business faces ongoing scrutiny from animal welfare groups and regulatory pushes for alternative methods. This is a reputational risk that requires proactive management and investment in NAMs.
Strategic Recommendations
Quick Wins
- Recommendation:
Launch targeted digital marketing campaigns highlighting integrated Cell & Gene Therapy case studies.
Expected Impact:Medium
Implementation Difficulty:Easy
Description:Showcase successful projects that moved seamlessly from viral vector production to preclinical testing to clinical manufacturing, emphasizing speed and reduced risk compared to using multiple vendors.
- Recommendation:
Develop and promote content assets (webinars, eBooks) specifically for AI-first biotech companies.
Expected Impact:Medium
Implementation Difficulty:Moderate
Description:Position CRL as the essential partner for 'in vivo' and 'in vitro' validation of digitally discovered drug candidates, creating a new sales funnel from this growing sector.
Medium Term Strategies
- Recommendation:
Expand flexible, modular manufacturing capacity (e.g., 'flex platforms') for personalized medicines.
Expected Impact:High
Implementation Difficulty:Difficult
Description:Invest in facilities and platforms designed for smaller, faster batches to capture the growing pipeline of cell therapies, gene therapies, and other personalized treatments beyond standard CAR-T.
- Recommendation:
Formalize a strategic partnership program with leading AI drug discovery companies.
Expected Impact:High
Implementation Difficulty:Moderate
Description:Move beyond a simple vendor relationship to co-develop integrated workflows, offering preferred access to preclinical testing in exchange for being the partner of choice for their pipelines.
Long Term Strategies
- Recommendation:
Become the market leader in services for emerging therapeutic modalities (e.g., mRNA, CRISPR-based therapies, radiopharmaceuticals).
Expected Impact:High
Implementation Difficulty:Difficult
Description:Systematically build or acquire the unique discovery, manufacturing, and analytical capabilities required for the next wave of therapeutic innovations to stay ahead of the competitive curve.
- Recommendation:
Develop a data-driven service layer across the portfolio.
Expected Impact:High
Implementation Difficulty:Difficult
Description:Leverage the vast amount of data generated from decades of studies to offer predictive insights on toxicology, manufacturability, and clinical success, creating a new value proposition that competitors cannot easily replicate.
Double down on the positioning as the indispensable, science-led partner for complex, high-stakes drug development. Shift the narrative from a 'collection of services' to a 'fully integrated journey,' emphasizing risk reduction, scientific collaboration, and accelerated pathways to regulatory approval.
Differentiate on the basis of unparalleled breadth and depth. While competitors may be strong in specific pillars (clinical trials, manufacturing, products), CRL's key differentiator is its ability to seamlessly integrate world-class capabilities across the entire R&D continuum, from research models to market.
Whitespace Opportunities
- Opportunity:
IND-in-a-Box for Virtual Biotechs
Competitive Gap:Small and startup biotechs often lack the broad expertise to manage the complex process of moving from discovery to an IND filing. No single competitor offers a fully managed, turnkey service package for this segment.
Feasibility:High
Potential Impact:High
Description:Create a bundled, milestone-based service offering that shepherds a client's lead candidate through all necessary preclinical safety, toxicology, and CMC (Chemistry, Manufacturing, and Controls) activities required for a successful IND submission.
- Opportunity:
Specialized preclinical services for validating AI-discovered targets and molecules.
Competitive Gap:AI discovery firms generate novel hypotheses at a rapid pace but need robust, fast, and reliable biological validation. Existing CRO offerings are not always tailored to the unique nature and speed of these clients.
Feasibility:Medium
Potential Impact:High
Description:Develop high-throughput screening and validation platforms specifically designed to test candidates from computational models, positioning CRL as the 'gold standard' validation engine for the AI drug discovery ecosystem.
- Opportunity:
Cryopreservation and Biologistics as a Strategic Service
Competitive Gap:While many companies offer cryopreservation, few integrate it strategically with cell therapy development and manufacturing. There's a gap in providing end-to-end cold chain logistics and long-term storage tied directly to CDMO services.
Feasibility:Medium
Potential Impact:Medium
Description:Expand cryopreservation services beyond just genetically engineered models to become a strategic hub for storing and managing critical starting materials and final drug products for cell and gene therapy clients.
Charles River Laboratories operates within a mature, moderately concentrated, and high-barrier-to-entry CRO/CDMO market. Its primary competitive advantage lies in its uniquely integrated, end-to-end portfolio that spans the entire drug development lifecycle, from foundational research models—a segment it dominates—through to commercial manufacturing. This integration is a powerful differentiator against competitors who are often specialized in specific segments. For instance, Labcorp and IQVIA are powerhouses in clinical trials and data, but lack CRL's deep preclinical and discovery foundation. Conversely, CDMO specialists like Lonza are manufacturing experts but do not offer the early-stage research services.
The most significant competitive threat comes from Thermo Fisher Scientific, which, through its acquisitions of PPD and Patheon, has assembled a similarly broad portfolio, further augmented by its dominant position in lab products and equipment. CRL's key defense and primary point of differentiation is its deep-rooted scientific identity and collaborative approach, positioning itself not just as a service provider, but as a fundamental R&D partner.
Key industry trends, such as the explosion in cell and gene therapies and the rise of AI in drug discovery, represent both the greatest opportunities and threats. CRL is well-positioned to capture growth in advanced therapies, as evidenced by its content and services around lentiviral vectors and CAR-T. The rise of AI-driven biotech presents a whitespace opportunity to become the preferred partner for biological validation. Strategic success will depend on CRL's ability to maintain its scientific edge, successfully integrate its diverse services to provide a seamless client experience, and make targeted investments in next-generation therapeutic modalities to preempt competitive encroachment. The company's primary weaknesses—the complexity of its vast operations and a perception of premium pricing—must be managed by reinforcing the value proposition of de-risking and accelerating the path to market.
Messaging
Message Architecture
Key Messages
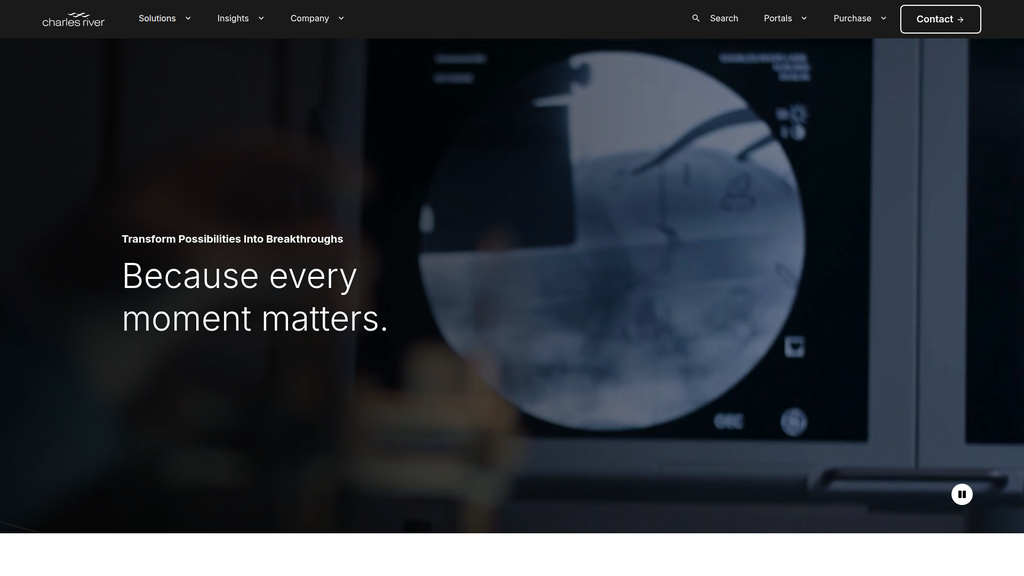
- Message:
We are the essential end-to-end partner for the entire drug discovery and development continuum.
Prominence:Primary
Clarity Score:High
Location:Homepage - 'Supporting the full drug discovery and development continuum'
- Message:
Our partnership transforms scientific possibilities into life-saving breakthroughs.
Prominence:Secondary
Clarity Score:High
Location:Homepage - Headline 'Transform Possibilities Into Breakthroughs'
- Message:
We are a proven, market-leading authority, integral to the majority of FDA-approved drugs.
Prominence:Secondary
Clarity Score:High
Location:Homepage - Statistic '80% Of the FDA-approved drugs over the past five years were worked on by Charles River'
- Message:
We are a purpose-driven organization focused on creating healthier lives.
Prominence:Tertiary
Clarity Score:Medium
Location:Homepage - 'Our people are the heart of our organization...achieving our purpose of creating healthier lives.'
The messaging hierarchy is strategically sound. It begins with a broad, aspirational vision ('Transform Possibilities') and immediately supports it with a powerful and unique proof point (80% of FDA drugs). The core value proposition of being an 'end-to-end' partner is then clearly articulated and broken down into specific service phases. This structure effectively caters to both high-level decision-makers and technical users.
Messaging is highly consistent across the homepage and the technical blog content. The themes of scientific expertise, partnership, and enabling breakthroughs are woven throughout. For example, the homepage's claim of expertise is substantiated by the deep scientific dive in the 'Leveraging Lentiviral Vectors' article and its embedded call-to-action to 'Consult an Expert'.
Brand Voice
Voice Attributes
- Attribute:
Authoritative & Expert
Strength:Strong
Examples
- •
Supporting the full drug discovery and development continuum
- •
A position of responsibility
- •
Why Kenvue Trusts Our Microbial Testing for Product Release
- •
Leveraging Lentiviral Vectors
- Attribute:
Purpose-Driven & Compassionate
Strength:Moderate
Examples
- •
Because every moment matters.
- •
Hope for the Future
- •
patients and their families desire one thing above all: more time.
- •
creating healthier lives
- Attribute:
Collaborative
Strength:Moderate
Examples
- •
we are driven to partner with Disruptors
- •
Join our team
- •
Collaborating to Cure Neurodegenerative Diseases
- Attribute:
Professional & Formal
Strength:Strong
Examples
Maximize Safer, Targeted Biologic Development...
USP <73> and USP <1071>: Accelerating Contamination Detection...
Tone Analysis
Scientific Authority
Secondary Tones
- •
Inspirational
- •
Collaborative
- •
Educational
Tone Shifts
The tone shifts from inspirational and purpose-driven in the homepage hero section to a more functional, descriptive tone in the service descriptions.
The 'Eureka' blog section adopts a deeply technical and educational tone, appropriate for its scientific audience.
Voice Consistency Rating
Excellent
Consistency Issues
No itemsValue Proposition Assessment
Charles River is the essential, end-to-end scientific partner that accelerates and de-risks the entire drug development journey, from basic research to commercialization, leveraging an unparalleled portfolio and a proven track record of success with the majority of FDA-approved drugs.
Value Proposition Components
- Component:
Comprehensive, End-to-End Portfolio
Clarity:Clear
Uniqueness:Somewhat Unique
- Component:
Unmatched Market Experience & Success
Clarity:Clear
Uniqueness:Unique
- Component:
Deep Scientific & Regulatory Expertise
Clarity:Clear
Uniqueness:Common
- Component:
Collaborative Partnership Model
Clarity:Somewhat Clear
Uniqueness:Somewhat Unique
The primary differentiator is the powerful social proof of having worked on '80% Of the FDA-approved drugs'. This is a highly specific, quantifiable, and impactful claim that competitors would find difficult to match. While many Contract Research Organizations (CROs) claim end-to-end services, Charles River's statistic powerfully substantiates its claim to leadership and de-risking for clients. The 'Disruptors' campaign adds a unique narrative layer to the common 'partnership' message.
The messaging positions Charles River not just as a service provider, but as the central, indispensable partner in the biopharmaceutical ecosystem. It messages a position of market dominance, stability, and unparalleled experience, appealing to clients who want to minimize risk and maximize the probability of success. They are positioned as the industry standard.
Audience Messaging
Target Personas
- Persona:
Biopharma Executive / C-Suite Decision-Maker
Tailored Messages
- •
80% Of the FDA-approved drugs over the past five years were worked on by Charles River
- •
Supporting the full drug discovery and development continuum
- •
Bringing and sustaining vital drugs to market safely, and as quickly as possible.
Effectiveness:Effective
- Persona:
Scientist / R&D Professional
Tailored Messages
- •
eBook: Exploratory Toxicology for Neuroscience Drug Discovery
- •
Leveraging Lentiviral Vectors
- •
Maximize Safer, Targeted Biologic Development with Smarter NAMs-Based Off-Target Screening
Effectiveness:Effective
- Persona:
Potential Employee / Talent
Tailored Messages
- •
This is Your Moment
- •
Our people are the heart of our organization.
- •
We strive to create a culture of belonging, continuous learning, and wellbeing...
Effectiveness:Effective
Audience Pain Points Addressed
- •
The complexity of the drug development process.
- •
The risk of failure in clinical trials.
- •
The need for specialized scientific expertise and technology.
- •
The pressure to accelerate time-to-market.
- •
The challenge of navigating regulatory requirements.
Audience Aspirations Addressed
- •
Developing a breakthrough, life-saving therapy.
- •
Curing devastating diseases like neurodegenerative disorders.
- •
Successfully bringing a drug to market.
- •
Making a meaningful impact on global health.
Persuasion Elements
Emotional Appeals
- Appeal Type:
Hope
Effectiveness:High
Examples
Hope for the Future
Transform Possibilities Into Breakthroughs
- Appeal Type:
Urgency / Purpose
Effectiveness:High
Examples
Because every moment matters.
When it comes to neurodegenerative diseases, patients and their families desire one thing above all: more time.
Social Proof Elements
- Proof Type:
Quantifiable Achievement
Impact:Strong
- Proof Type:
Expert Authority
Impact:Strong
- Proof Type:
Customer Case Studies
Impact:Moderate
Trust Indicators
- •
Specific, verifiable data points ('80%', '1,500 IND studies')
- •
Named customer case studies ('Why Kenvue Trusts...')
- •
PhD-credentialed authors on technical content
- •
Extensive library of scientific resources (webinars, eBooks)
- •
Long-standing industry presence (implied)
Scarcity Urgency Tactics
None observed, which is appropriate for the industry's long sales cycles and relationship-based model.
Calls To Action
Primary Ctas
- Text:
Meet the Disruptors
Location:Homepage Hero
Clarity:Clear
- Text:
Join our team
Location:Homepage Mid-page
Clarity:Clear
- Text:
Explore the Portfolio
Location:Basic Research Section
Clarity:Clear
- Text:
Consult an Expert
Location:Blog Post
Clarity:Clear
- Text:
Watch On Demand
Location:Blog Post
Clarity:Clear
The CTAs are contextually relevant and clear. They effectively segment the audience, guiding potential clients towards deeper content ('Explore the Portfolio'), brand stories ('Meet the Disruptors'), or direct contact ('Consult an Expert'). The use of 'Consult an Expert' on technical content is particularly strong, as it directly aligns with the user's mindset in that context. However, there is an opportunity to make the primary conversion-focused CTAs more prominent and benefit-driven across the main service pages.
Messaging Gaps Analysis
Critical Gaps
The customer's voice is underdeveloped. While a case study is present, weaving direct customer testimonials and success stories into the main service pages would add a powerful layer of validation.
There is no clear, branded methodology or process articulated. A 'Charles River Way' could codify their approach to partnership and execution, turning their process into a branded asset.
Contradiction Points
No significant contradictions were found. The messaging is remarkably consistent.
Underdeveloped Areas
The concept of 'partnership' is stated but could be more vividly demonstrated. Expanding the 'Disruptors' campaign to include more client-told stories would strengthen this pillar.
While expertise is clear, messaging around innovation and proprietary technologies could be more prominent to counter the perception of being just a large, established player.
Messaging Quality
Strengths
- •
Extraordinary use of a single, powerful data point ('80% of FDA-approved drugs') to anchor all claims of leadership and expertise.
- •
A masterful balance between a high-level, purpose-driven brand narrative and deep, credible scientific content.
- •
Effective storytelling through the 'Disruptors' campaign, which humanizes their work and highlights their collaborative ethos.
- •
Clear audience segmentation, providing distinct content paths for executives, scientists, and potential employees.
Weaknesses
The core value proposition, while implied, is not stated in a single, concise, and memorable sentence on the homepage.
CTAs, while clear, could be more consistently benefit-oriented and action-driven (e.g., 'Accelerate Your Research' vs. 'Explore the Portfolio').
Opportunities
- •
Feature client partners more prominently as the heroes of the story, with Charles River as the essential guide, to enhance the partnership message.
- •
Develop interactive tools that help prospective clients navigate the vast portfolio and self-identify the solutions they need.
- •
Create thought leadership content that not only educates but also sets the future agenda for the industry, solidifying their position as a forward-thinking leader.
Optimization Roadmap
Priority Improvements
- Area:
Homepage Value Proposition
Recommendation:Craft a single, powerful summary statement that encapsulates the core value proposition and place it prominently below the main hero. For example: 'The essential partner for biopharma leaders, de-risking and accelerating the journey from discovery to market.'
Expected Impact:High
- Area:
Customer-Centric Storytelling
Recommendation:Integrate short, impactful client testimonials and logos directly into the service pages to provide immediate social proof at the point of consideration.
Expected Impact:High
- Area:
Call-to-Action Strategy
Recommendation:On key service pages, prioritize a single, high-intent CTA like 'Schedule a Consultation' or 'Discuss Your Project' to streamline the path to lead generation.
Expected Impact:Medium
Quick Wins
- •
Re-word the 'Explore the Portfolio' CTA to be more benefit-focused, such as 'Find Your Solution'.
- •
Add the '80%' statistic to the header or footer of the site for persistent visibility.
- •
Create a short video version of the Kenvue case study for the homepage.
Long Term Recommendations
Develop a comprehensive thought leadership platform around the 'Disruptors' theme, featuring podcasts, interviews, and co-authored papers with innovative clients.
Formalize and brand the Charles River methodology for project management and client partnership, using it as a key differentiator in sales and marketing materials.
Charles River Laboratories executes a world-class strategic messaging strategy that effectively positions it as the undisputed market leader in the CRO industry. The messaging architecture is built on a foundation of unassailable quantitative proof—the '80% of FDA-approved drugs' statistic is a masterstroke that validates every other claim of expertise, reliability, and scale. The brand voice is expertly calibrated, blending scientific authority with a compelling, purpose-driven narrative that appeals to both the rational and emotional drivers of its target audiences.
The value proposition is clear and powerfully differentiated, focusing on de-risking the complex, high-stakes process of drug development. The content successfully segments its audience, serving high-level strategic messaging to decision-makers while providing deep, technical substance for scientific professionals. Persuasion is achieved not through aggressive tactics but through an overwhelming demonstration of credibility, expertise, and shared purpose.
While exceptionally strong, the messaging could be further optimized. The core value proposition, though evident, should be explicitly and concisely stated on the homepage to maximize immediate impact. The narrative, while telling a great story about partnership, could be made even more powerful by elevating the customer's voice through more prominent testimonials and client-centric stories. By implementing these refinements, Charles River can solidify its position not just as a service provider, but as the essential, defining partner in creating the future of medicine.
Growth Readiness
Growth Foundation
Product Market Fit
Strong
Evidence
- •
Market Leadership: Charles River is a critical partner to the global biopharmaceutical industry, having worked on 80% of FDA-approved drugs in the past five years.
- •
Comprehensive Portfolio: The company offers an end-to-end suite of services covering the entire drug discovery and development continuum, from basic research to manufacturing support.
- •
High Client Penetration: Serves 100% of the top 50 pharmaceutical companies, indicating deep integration and trust within its primary customer segment.
- •
Indispensable Role: Provides essential, non-discretionary services (e.g., safety and toxicology testing, research models) that are fundamental to drug development and regulatory approval.
Improvement Areas
Articulate a clearer value proposition for small to mid-sized biotech companies, which may require more flexible or bundled service offerings.
Enhance integration and cross-selling between its Discovery, Safety Assessment, and Manufacturing segments to increase wallet share with existing clients.
Market Dynamics
The Contract Research Organization (CRO) market is projected to grow at a CAGR of 6.9% to 8.27%, while the CDMO market is growing at a CAGR of around 9.0%.
Mature
Market Trends
- Trend:
Increased outsourcing of R&D and manufacturing by biopharma companies to reduce costs and accelerate timelines.
Business Impact:Positive: Sustained, high-quality demand for CRL's core services.
- Trend:
Growth in complex biologics, especially cell and gene therapies (CGTs), requiring specialized manufacturing capabilities.
Business Impact:High-Growth Opportunity: Creates demand for CRL's specialized CDMO services, but also presents significant scalability and technical challenges.
- Trend:
Rise of personalized medicine and targeted therapies.
Business Impact:Positive: Drives demand for more specialized research models and complex preclinical testing services.
- Trend:
Regulatory push for New Approach Methodologies (NAMs) to reduce reliance on animal testing.
Business Impact:Strategic Threat & Opportunity: Requires significant investment in alternative testing models (e.g., in vitro, computational) to adapt its core Safety Assessment business.
- Trend:
Adoption of AI and machine learning in drug discovery and development.
Business Impact:Opportunity: Potential to enhance service offerings, improve operational efficiency, and create new value-added services.
Excellent: The biopharmaceutical industry's structural shift towards outsourcing is a powerful and durable tailwind for CRL's business model.
Business Model Scalability
Medium
High fixed costs associated with global laboratory facilities, specialized equipment, and skilled personnel. Variable costs are tied to specific client projects.
Moderate. As a services and manufacturing business, scaling requires significant capital expenditure and talent acquisition, limiting purely digital scalability. However, its global footprint provides some leverage.
Scalability Constraints
- •
Capital Intensity: Expansion of laboratory and manufacturing capacity requires significant upfront investment.
- •
Talent Acquisition: Access to a highly specialized scientific workforce (e.g., Ph.D. level scientists, cell therapy manufacturing specialists) is a key constraint.
- •
Regulatory Compliance: Scaling operations across multiple international jurisdictions adds significant complexity and cost.
- •
Supply Chain: Sourcing of critical materials, particularly for advanced therapies, can be a bottleneck.
Team Readiness
Strong. As a long-established public company, CRL has an experienced leadership team adept at managing a large, global organization. Recent board changes aim to enhance strategic agility and capital allocation.
Segmented (Research Models & Services, Discovery & Safety Assessment, Manufacturing Solutions). This structure allows for specialized focus but may create silos that hinder cross-selling.
Key Capability Gaps
- •
Computational Biology and AI: Deeper expertise is needed to integrate AI-driven services into the discovery and preclinical workflow.
- •
Digital Transformation Leadership: Leadership focused on digitizing processes and data management to improve efficiency and create new data-driven products.
- •
Commercialization for Small Biotechs: A dedicated team or business unit focused on the unique needs and buying cycles of venture-backed biotech firms.
Growth Engine
Acquisition Channels
- Channel:
Direct Enterprise Sales
Effectiveness:High
Optimization Potential:Medium
Recommendation:Implement a key account management (KAM) strategy focused on expanding relationships within the top 50 pharma clients to drive adoption of the full service portfolio.
- Channel:
Content Marketing & Thought Leadership
Effectiveness:High
Optimization Potential:High
Recommendation:Develop interactive content and ROI calculators tailored to specific therapeutic areas (e.g., oncology, neuroscience) to move prospects from awareness to consideration more efficiently.
- Channel:
Industry Events & Conferences
Effectiveness:High
Optimization Potential:Medium
Recommendation:Focus presence on specialized, high-growth conferences (e.g., cell & gene therapy, AI in drug discovery) rather than just broad industry events to target innovators.
- Channel:
Referrals & Partnerships
Effectiveness:Medium
Optimization Potential:High
Recommendation:Build formal partnership programs with venture capital firms, academic incubators, and specialized technology providers to generate a pipeline of early-stage biotech clients.
Customer Journey
A long, high-touch, consultative sales process, moving from initial scientific inquiry to complex project scoping, proposal, and contract negotiation.
Friction Points
- •
Complexity in project scoping and pricing for novel therapies.
- •
Lengthy legal and procurement cycles within large pharmaceutical clients.
- •
Difficulty for smaller biotechs in navigating CRL's extensive and complex service portfolio.
Journey Enhancement Priorities
{'area': 'Onboarding for Small Biotechs', 'recommendation': "Create a 'Biotech Concierge' service or a streamlined digital portal for early-stage companies to access standardized services and expertise more easily."}
{'area': 'Proposal & Scoping Process', 'recommendation': 'Develop modular service packages and interactive scoping tools to accelerate the proposal generation and contracting phases.'}
Retention Mechanisms
- Mechanism:
End-to-End Service Portfolio
Effectiveness:High
Improvement Opportunity:Proactively map out a client's full development journey and offer integrated service bundles that create high switching costs and long-term value.
- Mechanism:
Master Service Agreements (MSAs)
Effectiveness:High
Improvement Opportunity:Incorporate incentives and preferred pricing within MSAs for clients that commit to using a broader range of CRL services.
- Mechanism:
Scientific & Regulatory Expertise
Effectiveness:High
Improvement Opportunity:Establish a formal 'Scientific Advisory Board' for key clients to deepen the partnership beyond a transactional vendor relationship.
Revenue Economics
Highly Favorable. While Customer Acquisition Cost (CAC) is high due to the enterprise sales model, the Lifetime Value (LTV) of a biopharma partner is exceptionally high, often spanning many years and multiple projects worth millions of dollars.
Estimated to be very strong (likely >10:1) given the long-term, high-value nature of client relationships.
High. Strong reputation and market leadership position create an efficient revenue engine, though it is capital and labor-intensive.
Optimization Recommendations
- •
Focus on 'land and expand' strategies to increase LTV of existing customers by cross-selling higher-margin services like manufacturing.
- •
Develop lower-touch, digitally-enabled acquisition funnels for smaller, more standardized service offerings to reduce CAC for that segment.
- •
Optimize resource allocation and project management to improve gross margins on service delivery.
Scale Barriers
Technical Limitations
- Limitation:
Manufacturing Capacity for Advanced Therapies
Impact:High
Solution Approach:Aggressively invest in expanding cGMP manufacturing facilities for cell and gene therapies through both greenfield projects and strategic acquisitions of specialized CDMOs.
- Limitation:
Data Integration and Analytics
Impact:Medium
Solution Approach:Develop a unified data platform to aggregate preclinical and manufacturing data, enabling AI/ML applications and providing clients with deeper insights.
Operational Bottlenecks
- Bottleneck:
Talent Acquisition and Retention
Growth Impact:Severe constraint on scaling specialized service areas.
Resolution Strategy:Establish strategic partnerships with leading academic institutions, develop advanced internal training programs, and create a compelling employer brand within the scientific community.
- Bottleneck:
Global Regulatory & Compliance Management
Growth Impact:Slows down international expansion and service deployment.
Resolution Strategy:Invest in a centralized regulatory intelligence platform and build dedicated regional expert teams to navigate diverse international standards efficiently.
- Bottleneck:
Project Management at Scale
Growth Impact:Can lead to margin erosion and decreased client satisfaction if not managed effectively.
Resolution Strategy:Implement next-generation project and resource management software, potentially leveraging AI for forecasting and scheduling.
Market Penetration Challenges
- Challenge:
Intense Competition
Severity:Critical
Mitigation Strategy:Differentiate through scientific expertise in high-growth niches (e.g., cell therapy, neuroscience), unparalleled quality, and by offering a truly integrated end-to-end solution. Competitors include LabCorp, IQVIA, and WuXi AppTec.
- Challenge:
Client Consolidation
Severity:Major
Mitigation Strategy:Diversify the client base by aggressively targeting the fragmented and innovative small/mid-cap biotech sector. Strengthen partnerships to become an indispensable part of their R&D process.
- Challenge:
Pricing Pressure
Severity:Major
Mitigation Strategy:Shift the value proposition from being a cost-saving vendor to a strategic partner that accelerates time-to-market and de-risks development, justifying premium pricing.
Resource Limitations
Talent Gaps
- •
Cell & Gene Therapy Manufacturing Scientists/Technicians
- •
Computational Toxicologists and Bioinformaticians
- •
AI/ML Data Scientists with a biology background
- •
Strategic Account Managers with deep scientific expertise
High. Continuous investment is required for facility expansion, technology upgrades (e.g., automation, single-use systems), and potential strategic acquisitions to enter new technology areas.
Infrastructure Needs
- •
Expanded cGMP manufacturing suites for viral vectors and cell therapies.
- •
Modernized, cloud-based data infrastructure for managing large-scale genomic and toxicology datasets.
- •
Automation and robotics in laboratories to increase throughput and reduce costs.
Growth Opportunities
Market Expansion
- Expansion Vector:
Deeper Penetration of Small/Mid-Sized Biotech Sector
Potential Impact:High
Implementation Complexity:Medium
Recommended Approach:Create a dedicated business unit with flexible service models, pre-vetted service packages, and financing-friendly payment structures tailored to venture-backed companies.
- Expansion Vector:
Geographic Expansion in APAC Biotech Hubs
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Pursue strategic acquisitions of established local CROs/CDMOs in key markets like China, South Korea, and Japan to gain immediate market access and local regulatory expertise.
Product Opportunities
- Opportunity:
Expansion of Cell & Gene Therapy (CGT) CDMO Services
Market Demand Evidence:The CGT market is a high-growth segment of the biopharma industry, with a well-documented manufacturing bottleneck creating huge demand for reliable CDMOs.
Strategic Fit:Perfect. Leverages CRL's core expertise in preclinical development and manufacturing, moving them up the value chain.
Development Recommendation:Pursue a 'build and buy' strategy: acquire smaller, specialized CGT CDMOs for their technology and talent, while simultaneously investing in large-scale greenfield manufacturing sites.
- Opportunity:
AI-Powered Preclinical Services
Market Demand Evidence:Biopharma companies are heavily investing in AI to make drug discovery and development faster and more predictive.
Strategic Fit:Excellent. Combines CRL's vast proprietary preclinical dataset with AI to create predictive toxicology and discovery models, offering a significant value-add.
Development Recommendation:Form strategic partnerships with leading AI drug discovery companies. Build an internal team of computational biologists and data scientists to develop proprietary AI-based service offerings.
- Opportunity:
Cryopreservation and Biologistics Services
Market Demand Evidence:The rise of personalized cell therapies creates complex logistical challenges for collecting, storing, and transporting patient-specific materials globally.
Strategic Fit:Strong. An adjacent service that leverages CRL's global footprint and quality control expertise, creating a stickier customer relationship.
Development Recommendation:Acquire or partner with a specialized cryogenic logistics provider to integrate these services into the cell therapy manufacturing workflow.
Channel Diversification
- Channel:
Digital Self-Service Platform
Fit Assessment:Good for standardized services.
Implementation Strategy:Develop a secure client portal for ordering standardized research models, microbial testing kits, or simple toxicology screens. This would cater to academic labs and small biotechs, freeing up the sales team for complex, high-value deals.
Strategic Partnerships
- Partnership Type:
Technology Integration
Potential Partners
- •
Schrodinger
- •
Recursion Pharmaceuticals
- •
BenevolentAI
Expected Benefits:Integrate cutting-edge AI discovery platforms with CRL's preclinical testing services to offer a seamless 'discovery-to-IND' package for clients.
- Partnership Type:
Venture Capital & Incubators
Potential Partners
- •
Flagship Pioneering
- •
Third Rock Ventures
- •
Y Combinator (Bio)
Expected Benefits:Become the preferred CRO/CDMO partner for their portfolio companies, gaining early access to the most promising new therapies and building long-term relationships.
- Partnership Type:
Academic Collaborations
Potential Partners
- •
MIT
- •
Stanford
- •
Max Planck Institutes
Expected Benefits:Gain access to novel technologies and top-tier scientific talent. Co-develop new research models and testing methodologies to stay at the scientific forefront.
Growth Strategy
North Star Metric
Number of Client Programs Advanced to a Key Development Milestone (e.g., IND Filing)
This metric directly aligns with CRL's mission of bringing drugs to market. It measures not just activity (revenue) but client success and progress, reflecting CRL's role as a strategic partner. It captures value across all business segments.
Increase by 15% year-over-year, focusing on high-value programs in biologics and advanced therapies.
Growth Model
Hybrid: Sales-Led Growth + Thought Leadership
Key Drivers
- •
Scientific Reputation & Quality
- •
Deep Enterprise Relationships (Key Account Management)
- •
Breadth and Integration of Service Portfolio
- •
Strategic Content demonstrating expertise in cutting-edge science
Double down on the enterprise sales model for large pharma. Simultaneously, build a scalable, content-driven inbound engine to attract and nurture leads from the burgeoning biotech sector, transitioning them to a specialized 'biotech sales' team.
Prioritized Initiatives
- Initiative:
Aggressive Expansion of Cell & Gene Therapy CDMO Capacity
Expected Impact:High
Implementation Effort:High
Timeframe:24-36 months
First Steps:Initiate a strategic review to identify and acquire a specialized viral vector or cell therapy CDMO within the next 6-9 months.
- Initiative:
Launch 'CRL Catalyst': A Service Platform for Emerging Biotechs
Expected Impact:Medium
Implementation Effort:Medium
Timeframe:12-18 months
First Steps:Pilot a program with 5-10 VC-backed biotechs to define a core offering of bundled discovery and preclinical services with standardized pricing and contracts.
- Initiative:
Develop a Predictive Toxicology Platform (ToxAI)
Expected Impact:High
Implementation Effort:High
Timeframe:18-24 months
First Steps:Form a dedicated data science team to begin cataloging and structuring historical toxicology data. Identify an AI technology partner for platform development.
Experimentation Plan
High Leverage Tests
- Test Name:
Bundled Service Pricing Model
Hypothesis:Offering a bundled price for Discovery + Safety Assessment services will increase the adoption of the full preclinical portfolio by 25%.
Metrics
- •
Adoption rate of bundled services
- •
Total contract value
- •
Client feedback
- Test Name:
Digital Lead Nurturing for Webinars
Hypothesis:A targeted email nurture sequence for webinar attendees will increase 'Sales Qualified Leads' (SQLs) from content marketing by 40%.
Metrics
- •
Content engagement rates
- •
MQL to SQL conversion rate
- •
Number of meetings booked
Utilize a combination of CRM data (e.g., Salesforce) for pipeline metrics and marketing automation analytics (e.g., Pardot/Marketo) for engagement data. Track initiatives against the North Star Metric.
Quarterly review of major strategic initiatives and monthly review of marketing and sales optimization experiments.
Growth Team
A centralized 'Strategic Growth' function reporting to the Chief Strategy Officer, with embedded experts from each business unit. This is not a traditional D2C growth team.
Key Roles
- •
Head of Corporate Strategy & M&A
- •
Director, Emerging Biotech Commercialization
- •
Head of Cell & Gene Therapy Business Unit
- •
Director, Digital & AI Solutions
- •
Market Intelligence Analyst
Build capabilities through a combination of strategic hiring of external experts (especially in AI and CGT), targeted acquisitions of technology/talent, and establishing a formal internal innovation program.
Charles River Laboratories is a well-entrenched market leader with a formidable growth foundation, characterized by strong product-market fit and deep integration into the biopharmaceutical value chain. The company's primary growth engine is a powerful, relationship-based enterprise sales model, buttressed by a reputation for scientific excellence.
The most significant growth opportunity lies in capitalizing on the industry's shift towards more complex biologics, particularly cell and gene therapies. This requires an aggressive strategy to expand their specialized CDMO capabilities, as this is where the market is growing fastest and where bottlenecks create significant demand. Capturing this opportunity is not just an option but a strategic imperative to maintain market leadership.
However, the company faces considerable scale barriers. The business model is capital and talent-intensive, and growth is constrained by the ability to build facilities and hire specialized scientists. The primary challenge is to navigate the operational complexities of scaling a high-touch, regulated business while simultaneously investing in future-facing technologies like AI and alternative testing models (NAMs) to preempt market shifts.
Recommended Growth Strategy:
1. Dominate High-Growth Niches: Aggressively invest (organically and via M&A) to become the undisputed leader in Cell & Gene Therapy manufacturing services.
2. Capture the Long Tail of Innovation: Systematically target the fragmented but highly innovative emerging biotech sector with a tailored service offering ('CRL Catalyst').
3. Digitize the Core: Develop AI-powered services to create a new, high-margin revenue stream and build a deep competitive moat based on proprietary data and predictive analytics.
Success will depend on CRL's ability to execute on these complex, capital-intensive initiatives while maintaining its core reputation for quality and scientific rigor. The company is well-positioned for steady growth, but transformative growth requires bold, strategic bets in these key areas.
Legal Compliance
Charles River Laboratories maintains a comprehensive and globally-oriented 'Global Privacy and Data Protection Policy'. The policy is detailed, clearly outlining the types of personal data collected (professional information, contact details, payment information, etc.), the purposes for collection (responding to inquiries, fulfilling services, marketing), and the legal basis for processing. It explicitly states that it does not sell personal data to third parties for direct marketing without consent. For data transfers outside the EEA, the company relies on EU Standard Contractual Clauses, demonstrating a clear mechanism for GDPR compliance. The policy also references key principles like 'Privacy by Design' and 'Privacy by Default', indicating a proactive approach to data protection. Overall, the privacy policy is robust, accessible from the website footer, and appears to meet the requirements of major international data protection laws.
The website has a 'Terms & Conditions' page, which is standard for a corporate site. It covers essential areas such as intellectual property rights (copyrights and trademarks), user conduct (prohibiting spam and unauthorized advertising), and disclaimers of liability. The terms clearly state that the website content is for informational purposes and should not be considered error-free, which is a crucial disclaimer for a company in a scientific field. It also includes a clause restricting the use of the company's name and logos without written permission. The document references the Privacy Statement, appropriately linking the two key legal policies. The terms are clearly written and enforceable, providing a solid legal framework for the use of the website.
Upon visiting the website, a cookie consent banner is prominently displayed. It provides clear options to 'Accept All Cookies', 'Reject All', or 'Cookie Settings'. This granular control is a best practice under GDPR and other modern privacy laws. The 'Cookie Settings' option allows users to manage their preferences for different categories of cookies (e.g., functional, performance, targeting). This implementation suggests that non-essential cookies are likely not loaded before user consent is obtained, which aligns with the principle of prior consent. The presence of a dedicated cookie policy, while sometimes separate, appears integrated into their broader privacy documentation, explaining the types of cookies used.
Charles River demonstrates a strong commitment to global data protection, commensurate with its operational footprint across North America, Europe, and Asia. The Global Privacy Policy explicitly addresses GDPR and mentions safeguards like Standard Contractual Clauses for international data transfers. The company provides a dedicated web form for individuals to submit requests related to their data under GDPR. The policy outlines the rights of data subjects (access, rectification, erasure, etc.) and establishes a framework for handling data processing agreements (DPAs) with third parties, correctly identifying its potential roles as a Data Controller or Processor. This structured approach to data governance is a significant strength, reducing the risk of non-compliance with regulations like GDPR and CCPA/CPRA.
The website shows evidence of attention to accessibility. The inclusion of a 'Skip to main content' link in the site's code is a positive indicator of compliance with Web Content Accessibility Guidelines (WCAG). Navigating the site with a keyboard appears functional, and the general structure is logical. However, a full audit would be required to assess compliance with WCAG 2.1 Level AA standards, which is the common benchmark for B2B companies to avoid legal risk under laws like the Americans with Disabilities Act (ADA). While basic elements are in place, potential gaps could exist in areas like alt text for all images, video captions, and color contrast ratios across the entire site.
As a leading Contract Research Organization (CRO), Charles River's legal positioning is heavily dependent on its compliance with stringent industry-specific regulations. The website and company information clearly indicate adherence to Good Laboratory Practice (GLP) and Good Clinical Practice (GCP), which are foundational for preclinical and clinical research and required by regulatory bodies like the FDA in the US and EMA in the EU. The company's services are designed to help clients meet regulatory expectations for Investigational New Drug (IND) applications. Furthermore, as a provider of research models, the company is subject to animal welfare regulations, which represents a significant area of legal, ethical, and reputational risk that they must manage. Their public positioning emphasizes safety, quality, and regulatory compliance, which is essential for building trust and securing market access in the biopharmaceutical industry.
Compliance Gaps
- •
No explicit 'Do Not Sell or Share My Personal Information' link is immediately visible in the website footer, which is a specific requirement under the California Privacy Rights Act (CPRA). While their policy states they don't sell data, the link is a prescribed feature.
- •
The 'Terms & Conditions' document is dated 2017, which may predate significant regulatory changes like the full implementation of GDPR and the enactment of CCPA/CPRA. A review and update would be prudent.
- •
While basic accessibility features are present, a formal, public Accessibility Statement detailing the company's commitment and conformance level to WCAG standards is not readily apparent. This is becoming a standard best practice.
Compliance Strengths
- •
A comprehensive, globally-focused privacy policy that directly addresses GDPR requirements and international data transfers.
- •
A robust and granular cookie consent mechanism that provides users with clear choices and control.
- •
Clear communication of adherence to critical industry regulations like GLP and GCP, which is a core part of their value proposition.
- •
Provision of a dedicated online form for exercising GDPR data subject rights, demonstrating operational readiness for compliance.
- •
Strong governance framework for managing data with third parties, including the use of Data Processing Agreements.
Risk Assessment
- Risk Area:
CCPA/CPRA Compliance
Severity:Medium
Recommendation:Add a clear and conspicuous 'Do Not Sell or Share My Personal Information' link to the website footer to align with CPRA requirements, even if the company's policy is not to sell data. This mitigates risk of technical non-compliance.
- Risk Area:
Outdated Legal Documents
Severity:Low
Recommendation:Review and update the 'Terms & Conditions' to reflect the current regulatory landscape, including GDPR and CCPA/CPRA, and update the 'last revised' date to demonstrate ongoing legal oversight.
- Risk Area:
Website Accessibility (ADA/WCAG)
Severity:Low
Recommendation:Conduct a formal WCAG 2.1 AA audit of the website to identify and remediate any specific accessibility issues. Publish an Accessibility Statement to formalize the company's commitment and reduce potential legal exposure.
High Priority Recommendations
Implement a 'Do Not Sell or Share My Personal Information' link in the website footer to ensure full technical compliance with the California Privacy Rights Act (CPRA).
Conduct a formal accessibility audit against WCAG 2.1 AA standards and publish an Accessibility Statement to mitigate legal risks under the ADA and improve corporate social responsibility positioning.
Overall, Charles River Laboratories demonstrates a strong and mature legal compliance posture, which is a strategic asset given its role in the highly regulated global biopharmaceutical industry. The company's legal framework for data privacy is sophisticated, addressing the complexities of GDPR and international data flows, which is critical for a business with operations and clients across the US, Europe, and Asia. This builds significant customer trust, as their clients (pharmaceutical and biotech companies) must ensure their entire supply chain is compliant. Their public emphasis on adhering to industry-specific regulations like GLP and GCP is not just a legal requirement but a core competitive advantage that enables market access. The identified compliance gaps, such as the missing CPRA link and the need for a formal accessibility statement, are relatively minor and appear to be technical oversights rather than systemic failures. Addressing these low-effort, high-impact items would further solidify their position as a legally robust and trustworthy partner, reducing residual risk and enhancing their corporate reputation.
Visual
Design System
Corporate Scientific
Good
Developing
User Experience
Navigation
Horizontal Mega-Menu (Desktop) / Hamburger (Mobile)
Clear
Good
Information Architecture
Logical
Somewhat clear
Moderate
Conversion Elements
- Element:
Main Header 'Contact' Button
Prominence:Medium
Effectiveness:Somewhat effective
Improvement:Increase visual distinction from other non-conversion header links like 'Search' and 'Portal'. Consider a subtle but different color or background fill on hover.
- Element:
Sectional 'View All' / 'See All' Links
Prominence:Low
Effectiveness:Ineffective
Improvement:These links lack visual weight and resemble body text. Convert them to ghost buttons or add a prominent arrow icon to increase affordance and signal interactivity.
- Element:
'Join Our Team' CTA Button
Prominence:Medium
Effectiveness:Effective
Improvement:The solid dark background provides good contrast. The placement is logical within its section. No immediate improvement needed, but ensure consistency of this style for primary CTAs.
- Element:
Footer Contact Form/Link
Prominence:Low
Effectiveness:Somewhat effective
Improvement:The footer is dense. A more visually pronounced 'Contact Us' or 'Request Information' section header within the footer could improve visibility for users scrolling to the bottom for contact options.
Assessment
Strengths
- Aspect:
Professional & Trustworthy Brand Identity
Impact:High
Description:The visual design effectively communicates a sense of scientific precision, authority, and reliability. The clean typography, professional imagery, and structured layouts align perfectly with the expectations of its target audience in the pharmaceutical and biotech industries.
- Aspect:
Clear Primary Navigation
Impact:High
Description:The main navigation categories ('Solutions', 'Insights', 'Company') are logical and well-defined for a Contract Research Organization (CRO). This structure allows users from different segments (e.g., a scientist looking for a service vs. an investor looking at company info) to self-identify their journey easily.
- Aspect:
High-Quality, Relevant Imagery
Impact:Medium
Description:The website uses authentic, high-quality photography of scientists, labs, and abstract scientific concepts. This visual storytelling reinforces their expertise and avoids the generic feel of stock photography, building credibility and engagement.
Weaknesses
- Aspect:
Information-Dense Layouts
Impact:Medium
Description:Several sections, particularly the 'Supporting the full drug discovery...' area on the homepage and the body of the blog post, are text-heavy with insufficient whitespace and visual breaks. This increases cognitive load and can deter scannability, potentially causing users to miss key information.
- Aspect:
Inconsistent Call-to-Action (CTA) Design
Impact:High
Description:There is a lack of a clear, consistent visual language for CTAs. Some are solid buttons ('Join Our Team'), while others are simple text links ('View All', 'Learn about our research...'). This inconsistency reduces the overall effectiveness of conversion pathways and can confuse users about what is clickable versus what is purely informational text.
- Aspect:
Understated Interactive Elements
Impact:Medium
Description:The tabbed interface in the 'drug discovery continuum' section is a good idea for organizing content, but the active/inactive states have very low contrast. This makes it difficult for users to know which tab is selected and reduces the usability of a key interactive component on the homepage.
Priority Recommendations
- Recommendation:
Establish a Hierarchical CTA System
Effort Level:Low
Impact Potential:High
Rationale:Define and consistently apply 2-3 visual styles for CTAs (e.g., Primary: solid blue button; Secondary: ghost button; Tertiary: link with arrow icon). Applying this system globally will significantly improve user guidance, clarify desired actions, and increase lead generation effectiveness.
- Recommendation:
Increase Whitespace and Visual Pacing in Content
Effort Level:Medium
Impact Potential:Medium
Rationale:Break up long text blocks on pages like the blog and homepage sections. Introduce more subheadings, bullet points, pull quotes, and increase top/bottom margins for sections. This will improve readability, reduce user fatigue, and increase content engagement.
- Recommendation:
Enhance Visual Feedback for Interactive Elements
Effort Level:Low
Impact Potential:Medium
Rationale:Increase the color contrast and visual distinction for active/hover states on tabs, links, and navigation items. Clear and immediate visual feedback improves usability, reduces user uncertainty, and creates a more responsive and intuitive experience.
Mobile Responsiveness
Good
The site handles standard breakpoints well, with content reflowing predictably. The primary navigation collapses into a functional hamburger menu, and typography scales appropriately for readability.
Mobile Specific Issues
Tap targets for some text-based links are small, potentially leading to mis-taps.
Text-heavy sections become very long scrolls without visual anchors, which can be fatiguing on mobile.
Desktop Specific Issues
Large, high-resolution monitors may experience overly wide text blocks, which can hinder readability. Constraining the max-width of content containers would improve the experience on very large screens.
Strategic Visual & UX Assessment of criver.com
Charles River Laboratories (CRL) is a global Contract Research Organization (CRO) providing extensive research and development services to the pharmaceutical, biotechnology, and medical device industries. Its target audience consists of highly educated professionals—scientists, researchers, and executives—who value precision, credibility, and efficiency. The website serves as a critical digital front door, reflecting the company's scientific authority and facilitating complex B2B interactions that are service-based rather than direct e-commerce transactions.
1. Design System and Brand Identity:
The website projects a Corporate Scientific aesthetic, which is highly appropriate for the industry. The color palette, dominated by a deep blue, white, and grey, evokes professionalism, trust, and intelligence. The brand identity is expressed consistently in terms of color and typography, earning it a Good rating for consistency. However, the design system lacks full maturity (Developing). While core elements are in place, there's inconsistency in applying styles to interactive components like buttons and links, indicating a system that is either not fully defined or not strictly adhered to.
2. Visual Hierarchy and Information Architecture:
The homepage's visual hierarchy is generally effective at the top level. The hero section, with its compelling tagline "Because every moment matters," immediately establishes a high-stakes, purpose-driven tone. However, as the user scrolls, the hierarchy weakens. Sections like "Supporting the full drug discovery and development continuum" present a large amount of information with low visual contrast between interactive tabs and static text, leading to a Moderate cognitive load. The information architecture is logical, but the visual execution doesn't always guide the user effectively through the content's importance.
3. Navigation and User Flow:
The primary desktop navigation is a clear, conventional horizontal mega-menu, which works well for the breadth of CRL's offerings. It adapts cleanly to a standard hamburger menu on mobile. The user flow for discovery is Somewhat clear; a user can find a top-level service category easily, but navigating deeper into specific sub-services or finding related case studies or resources from a service page could be more intuitive. The primary friction in the user flow is the lack of prominent, guiding CTAs to direct users to the next logical step.
4. Mobile Responsiveness:
The site demonstrates a Good level of mobile responsiveness. Layouts reflow logically, text remains readable, and core functionality is maintained across devices. The primary issue on mobile is the magnification of the content density problem; sections that are text-heavy on desktop become extended passages of scrolling on mobile, with few visual breaks to maintain engagement.
5. Visual Conversion Elements:
This is the most significant area for improvement. For a B2B organization like CRL, conversions are actions like 'Contact Sales', 'Request a Quote', or 'Download a Whitepaper'. The current CTAs are inconsistent and often have low prominence. For example, critical 'View All' and 'Learn More' links are styled as plain text, making them easy to overlook. A clearly defined visual hierarchy for buttons (e.g., solid for primary actions, outline for secondary) is needed to guide users toward these conversion goals effectively.
6. Visual Storytelling and Content Presentation:
The site excels in using high-quality, relevant imagery that tells a story of innovation and expertise. The photography of real lab environments and professionals lends authenticity. However, the presentation of long-form content, such as the 'Leveraging Lentiviral Vectors' blog post, is a weakness. The lack of visual variety—such as pull quotes, styled blockquotes, or embedded videos—results in a wall of text that is intimidating and difficult to scan, diminishing the impact of the valuable content itself.
Discoverability
Market Visibility Assessment
Charles River Laboratories (CRL) projects exceptional brand authority as a premier Contract Research Organization (CRO). Their claim of contributing to 80% of FDA-approved drugs in the last five years is a powerful statement of market leadership and scientific credibility. The website's 'Eureka' blog and 'Insights' sections, featuring in-depth articles by PhD-level staff on complex topics like lentiviral vectors, firmly establish them as thought leaders. This content strategy targets a highly sophisticated audience of scientists and researchers, positioning CRL not just as a service provider, but as a scientific peer.
As a leading global CRO, CRL has high brand recognition. Their main competitors include Labcorp Drug Development, IQVIA, and PPD (Thermo Fisher Scientific). While branded search visibility is strong, the key competitive arena is in search results for specific, high-value services (e.g., 'preclinical toxicology services', 'cell and gene therapy CDMO'). In these niche areas, CRL must continuously defend its visibility against both large, full-service competitors and smaller, specialized CROs. Their broad portfolio is a strength but requires a sophisticated digital strategy to ensure visibility across all service lines.
The digital presence is expertly tuned for high-value B2B lead generation within a long and complex sales cycle. The strategy is not direct sales but strategic engagement. By offering high-value content such as webinars, eBooks, and scientific case studies, they capture the interest of their target audience (pharma and biotech R&D leaders) and funnel them towards 'Consult an Expert' calls-to-action. The potential is immense, as a single lead can result in a multi-million dollar, multi-year contract.
CRL's digital presence reflects its global operational footprint, with facilities in over 20 countries. The website lists international events, indicating a strategy to engage with key biotech hubs in North America, Europe, and Asia. Digital content can further penetrate these markets by addressing region-specific regulatory challenges and research trends, thereby attracting localized interest and demonstrating global expertise with local understanding.
Coverage is comprehensive and deep, spanning the entire drug discovery and development continuum from basic research to manufacturing support. The provided content on 'Leveraging Lentiviral Vectors' is a prime example of their expertise in cutting-edge fields like cell & gene therapy. This demonstrates an alignment with key industry growth trends, such as the boom in biologics and personalized medicine, which is critical for attracting clients working on next-generation therapeutics.
Strategic Content Positioning
Content is well-mapped to the scientific and procurement journey. Awareness-stage content ('Eureka' blog) attracts researchers with scientific questions. Consideration-stage content (webinars, case studies) provides detailed solutions and methodologies. Decision-stage content (service pages, 'Consult an Expert' forms) facilitates direct engagement. This structured approach effectively nurtures prospects from initial scientific inquiry to a commercial conversation.
CRL is already a strong thought leader, but there is an opportunity to dominate specific high-growth therapeutic areas. They could develop comprehensive digital 'Command Centers'—pillar content hubs for areas like Oncology, Neuroscience, or mRNA therapeutics. These hubs would consolidate all related articles, webinars, case studies, and expert interviews, creating an unparalleled resource that would rank highly in search and become a go-to reference for researchers in those fields.
While CRL's scientific content is top-tier, competitors are also strong. A key opportunity lies in creating content that addresses the business and operational challenges of drug development. This could include ROI calculators for outsourcing, guides on navigating multi-regional regulatory submissions, or content that directly compares the strategic benefits of an integrated CRO partner versus a multi-vendor approach. This would appeal to executive-level decision-makers focused on budget and timelines.
Brand messaging is exceptionally consistent. The core message of being an integrated, end-to-end partner that helps de-risk and accelerate drug development is reinforced across the entire digital presence. The tagline 'Transform Possibilities Into Breakthroughs' and the 'Disruptors' campaign effectively frame their role not just as a contractor, but as a pivotal partner in scientific innovation.
Digital Market Strategy
Market Expansion Opportunities
- •
Target emerging biotech hubs (e.g., Singapore, South Korea) with geo-targeted content campaigns that highlight CRL's global capabilities and understanding of local market dynamics.
- •
Develop dedicated content streams for high-growth modalities like cell & gene therapy, biologics, and AI-driven drug discovery, positioning CRL at the forefront of these innovations.
- •
Create content specifically for small to mid-sized biotech firms, addressing their unique challenges (e.g., funding constraints, need for regulatory guidance) and positioning CRL as a scalable partner for growth.
Customer Acquisition Optimization
- •
Implement an Account-Based Marketing (ABM) strategy, using their rich content library to create personalized content journeys for high-value target accounts at major pharmaceutical companies.
- •
Optimize service pages and expert articles for long-tail, high-intent keywords that capture researchers seeking solutions to very specific scientific problems, leading to higher quality leads.
- •
Develop interactive tools, such as a service-selector guide or a preclinical trial cost estimator, to engage potential clients and capture valuable lead data early in their decision process.
Brand Authority Initiatives
- •
Launch a 'CRL Scientific Fellows' digital program, formally showcasing and promoting their top internal scientists through video interviews, guest articles in major industry publications, and a dedicated series on the 'Eureka' blog.
- •
Systematically pursue digital PR opportunities by pitching their experts' commentary on breaking scientific news and regulatory changes, securing high-authority backlinks and media mentions.
- •
Produce an annual 'State of Drug Development' report, leveraging CRL's vast proprietary data to provide unique industry insights, creating a highly citable and linkable asset.
Competitive Positioning Improvements
- •
Create content that explicitly details the strategic advantages of their integrated, end-to-end service model, focusing on reduced timelines, streamlined data management, and lower risk of project failure.
- •
Develop comparison guides and frameworks that help potential clients evaluate CRO partners, framing the criteria in a way that highlights CRL's strengths (e.g., global footprint, breadth of services, regulatory track record).
- •
Feature more client success stories and video testimonials that quantify the impact of partnering with CRL, moving beyond capabilities to focus on tangible outcomes like accelerated IND filings or market approvals.
Business Impact Assessment
Market share growth can be indicated by tracking organic search visibility for a basket of high-value commercial keywords (e.g., 'GLP toxicology studies', 'CAR-T manufacturing services') against key competitors. An increasing share of voice for these terms correlates with capturing greater market interest.
Success should be measured by the generation of Marketing Qualified Leads (MQLs) from gated content (webinars, eBooks) and direct inquiries ('Consult an Expert'). Key metrics include cost per MQL, lead-to-opportunity conversion rate, and the attributed contract value of leads originating from the digital presence.
Brand authority is measured by the quality and quantity of backlinks from reputable scientific, academic (.edu), and government domains. Other key indicators include brand search volume, media mentions of CRL's research and experts, and engagement rates on professional networks like LinkedIn.
Benchmark against 3-5 key competitors (e.g., Labcorp, Lonza, WuXi AppTec) on the depth and breadth of content in strategic growth areas. Regularly assess competitor rankings for critical service keywords and analyze the sophistication of their digital funnels to ensure CRL maintains a competitive edge.
Strategic Recommendations
High Impact Initiatives
- Initiative:
Develop 'Therapeutic Area Command Centers'
Business Impact:High
Market Opportunity:Positions CRL as the definitive expert in the most lucrative and competitive R&D fields (e.g., Oncology, Cell & Gene Therapy). Aims to capture a dominant share of search traffic and leads for these high-value services.
Success Metrics
- •
1-3 ranking for primary therapeutic area keywords
- •
Increase in MQLs for related services
- •
Growth in backlinks from specialty research domains
- Initiative:
Launch a 'Scientific Partner' ABM Content Program
Business Impact:High
Market Opportunity:Moves beyond broad lead generation to targeted pipeline creation. Focuses marketing resources on securing high-value, long-term contracts from the largest pharmaceutical and biotech companies by providing bespoke, relevant insights.
Success Metrics
- •
Engagement rate from target accounts
- •
Number of meetings booked with key decision-makers
- •
Pipeline value generated from targeted accounts
- Initiative:
Leverage Proprietary Data for Industry-Leading Reports
Business Impact:Medium
Market Opportunity:Differentiates CRL from competitors by turning their vast operational data into unique, valuable insights. Establishes them as a data-driven leader and creates a powerful asset for digital PR and lead generation.
Success Metrics
- •
Number of downloads/registrations
- •
Media mentions and citations
- •
Backlinks from authoritative industry sources
Shift the digital narrative from being a premier Contract Research Organization to being the indispensable strategic partner for de-risking and accelerating therapeutic innovation. This positioning leverages CRL's key differentiator: an unparalleled, integrated portfolio that spans the entire development lifecycle. The digital presence must consistently communicate that choosing CRL is the most strategic decision a biotech or pharmaceutical company can make to maximize the probability of scientific and commercial success.
Competitive Advantage Opportunities
- •
Highlight the 'Power of the Portfolio': Create content that explicitly demonstrates how the integration of services (e.g., from discovery to safety assessment to manufacturing) creates efficiencies and reduces timelines that disconnected vendors cannot match.
- •
Amplify Scientific Bench Strength: Systematically use digital channels to turn their army of in-house PhDs and scientists into visible industry thought leaders, creating a moat of expertise that is difficult for competitors to replicate.
- •
Data as a Differentiator: Leverage aggregated, anonymized data from thousands of studies to publish trend reports and predictive insights, showcasing a level of market intelligence that only a leader of their scale can possess.
Charles River Laboratories has established a formidable digital market presence that reflects its status as a global leader in the CRO industry. The website and associated content effectively project scientific authority and align with the complex, high-value B2B customer journey. The content strategy, exemplified by expert-authored articles on cutting-edge topics, successfully positions the company as a credible scientific partner, which is paramount in an industry built on trust and expertise. Their digital presence serves as a powerful engine for lead generation, targeting the precise needs of researchers and program leaders within pharmaceutical and biotech companies.
The primary strategic opportunity is to sharpen this tool for competitive differentiation and market expansion. While strong across the board, the digital strategy can evolve from demonstrating broad competence to asserting decisive dominance in the most profitable and fastest-growing segments of the market, such as cell & gene therapy and biologics. By creating comprehensive content hubs ('Therapeutic Area Command Centers'), CRL can own the digital conversation in these key battlegrounds. Furthermore, by leveraging Account-Based Marketing (ABM) and transforming their vast internal data into public-facing intelligence reports, CRL can build deeper relationships with high-value clients and solidify its position not merely as a vendor, but as an essential strategic partner. The focus should be on digitally articulating the unique value of their integrated portfolio, creating a clear competitive advantage that translates directly to winning high-value, long-term contracts.
Strategic Priorities
Strategic Priorities
- Title:
Dominate the Cell & Gene Therapy (CGT) CDMO Market
Business Rationale:The analysis identifies the CGT market as the single largest growth driver in the biopharmaceutical industry, with a well-documented manufacturing bottleneck. Competitors are aggressively investing here. Failing to capture a leading share of this market represents the most significant strategic risk and missed opportunity.
Strategic Impact:This initiative transforms the Manufacturing Solutions segment into the company's primary growth engine, shifting the revenue mix towards higher-margin, cutting-edge services and establishing a dominant position in the next generation of therapeutics.
Success Metrics
- •
Increase in CGT CDMO market share by 5% within 24 months
- •
Revenue from Manufacturing Solutions grows to >30% of total company revenue
- •
Reduction in sales cycle for CGT manufacturing contracts
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Market Position
- Title:
Launch "CRL Catalyst": A Dedicated Service Platform for Emerging Biotechs
Business Rationale:The analysis highlights that while large pharma provides stable revenue, the majority of therapeutic innovation originates from the fragmented small and mid-sized biotech sector. These companies have unique needs (speed, flexibility, integrated support) that are not fully met by the current enterprise-focused model. This is a large, underserved market segment.
Strategic Impact:Creates a new, scalable revenue stream and diversifies the client base, reducing dependency on large pharma R&D budgets. This positions Charles River as the foundational partner for the entire biotech ecosystem, capturing value from innovative therapies at their inception.
Success Metrics
- •
Revenue from the emerging biotech segment increases by 40% year-over-year
- •
Acquisition of 50 new biotech clients through the Catalyst platform in the first year
- •
Number of client programs advanced from discovery to IND filing via bundled services
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Revenue Model
- Title:
Develop a Proprietary AI-Powered Predictive Analytics Service
Business Rationale:The company possesses decades of invaluable, proprietary preclinical data. The analysis identifies this as a critically underleveraged asset. Competitors and new entrants are moving towards AI-driven drug discovery, and CRL must lead, not follow, to avoid the commoditization of its core testing services.
Strategic Impact:This initiative creates a new, high-margin, data-as-a-service (DaaS) revenue stream. It builds a deep competitive moat that is nearly impossible to replicate, transforming the company's value proposition from providing test results to providing predictive insights that de-risk drug development.
Success Metrics
- •
Launch of a beta predictive toxicology platform (ToxAI) within 18 months
- •
Generation of a new revenue stream from data/analytics products
- •
Number of strategic partnerships with AI-first drug discovery companies
Priority Level:HIGH
Timeline:Long-term Vision (12+ months)
Category:Customer Strategy
- Title:
Formalize and Market the "Integrated Journey" Client Experience
Business Rationale:The company's single greatest competitive advantage is its fully integrated, end-to-end portfolio. However, the analysis suggests that the client experience can feel siloed across business units. Formalizing and operationalizing a seamless journey is critical to defending against competitors who offer point solutions or have their own integrated offerings (e.g., Thermo Fisher).
Strategic Impact:Transforms the core value proposition from a 'collection of services' into a branded, high-value strategic pathway. This increases customer lifetime value, creates significant switching costs, and provides a clear, defensible marketing message that justifies premium pricing.
Success Metrics
- •
Increase in multi-segment (e.g., DSA + Manufacturing) contracts by 20%
- •
Improvement in client 'Net Promoter Score' (NPS) for cross-functional projects
- •
Reduction in client onboarding time for multi-service programs
Priority Level:MEDIUM
Timeline:Strategic Initiative (3-12 months)
Category:Operations
- Title:
Establish Strategic Alliances with AI Drug Discovery Pioneers
Business Rationale:AI-powered drug discovery companies are identified as a disruptive force and a new ecosystem of high-value potential clients. They do not compete on services but require a 'gold standard' partner for preclinical validation. A strategic partnership model is needed to capture this emerging market before competitors do.
Strategic Impact:Positions Charles River as the essential 'in-vivo / in-vitro' validation engine for the AI drug discovery revolution. This creates a new, high-growth sales channel and ensures the company's relevance and indispensability in the future of R&D.
Success Metrics
- •
Establishment of 3-5 formal partnerships with leading AI discovery firms
- •
Pipeline value generated from AI partner referrals
- •
Co-development of at least one integrated 'AI-to-IND' service offering
Priority Level:MEDIUM
Timeline:Quick Win (0-3 months)
Category:Partnerships
Charles River must evolve from being the premier provider of outsourced services to becoming the indispensable strategic partner for developing next-generation therapeutics. This requires dominating the high-growth Cell & Gene Therapy manufacturing market while leveraging its unique data assets and integrated portfolio to capture the innovative biotech ecosystem.
The key competitive advantage to build is the 'Power of the Integrated Portfolio,' a seamless, data-driven journey from discovery to manufacturing that demonstrably accelerates timelines and de-risks development for complex therapies.
The primary growth catalyst is the aggressive expansion of specialized Cell & Gene Therapy CDMO (Contract Development and Manufacturing Organization) capacity and expertise to capture the immense, underserved demand in the market.