eScore
edwards.comThe eScore is a comprehensive evaluation of a business's online presence and effectiveness. It analyzes multiple factors including digital presence, brand communication, conversion optimization, and competitive advantage.
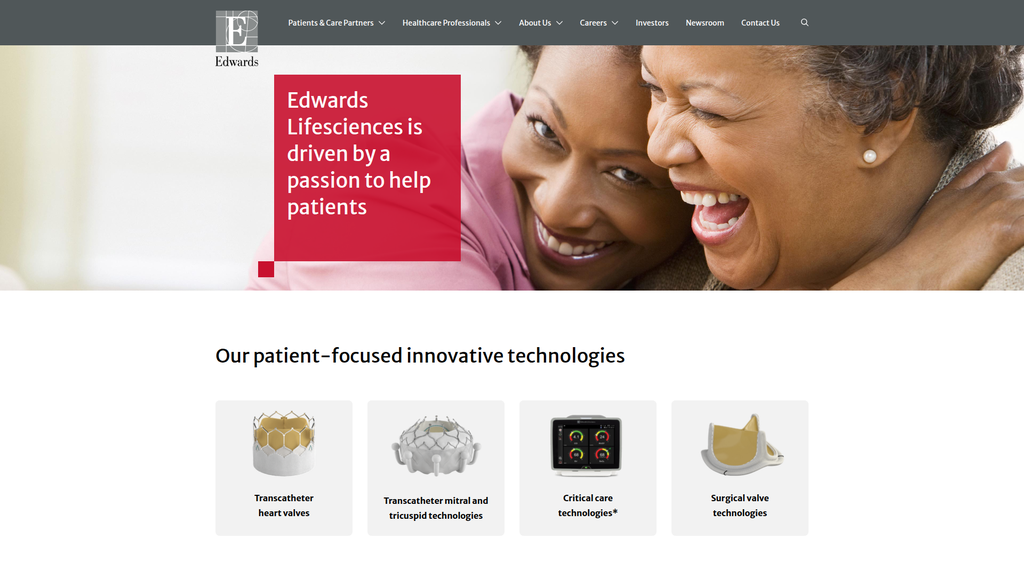
Edwards demonstrates exceptional search intent alignment by clearly segmenting content for its distinct 'Healthcare Professional' and 'Patient' audiences. Its digital authority is profound, built upon a deep well of clinical research and data that establishes it as a definitive resource in the structural heart space. However, its global digital reach is underdeveloped, with a primarily U.S.-centric website that lacks localized content for key international markets, representing a significant growth opportunity.
Excellent content authority and segmentation for distinct "Healthcare Professionals" and "Patient" audiences, ensuring high alignment with user search intent.
Develop region-specific content hubs and localized messaging for key international markets like Europe and Japan to improve global digital penetration and support in-country commercial teams.
The brand's core message, rooted in a 'passion to help patients,' is powerful, emotive, and consistently applied, creating a strong emotional connection. Audience segmentation in messaging is a clear strength. However, the communication is weakened by a failure to assert its documented market leadership and a critical lack of tangible social proof (patient stories, clinician testimonials) on the website to substantiate its claims.
The brand consistently and effectively uses an emotive, purpose-driven voice centered on a 'passion to help patients,' which resonates strongly with both patient and clinician audiences.
Revise homepage messaging to explicitly state its position as the 'global leader in structural heart innovation' and integrate a 'Patient Stories' section to provide the social proof needed to validate its emotional claims.
The website offers a highly professional and clean user experience with a low cognitive load and an excellent mobile-responsive design. This creates a smooth informational journey for users across devices. The score is significantly impacted by two major issues: the universal use of low-prominence text links instead of buttons for key calls-to-action, and a high-risk legal gap in website accessibility (WCAG compliance), which could alienate users with disabilities.
A seamless and 'Excellent' rated cross-device experience, combined with a well-organized information architecture, creates a light cognitive load and makes complex information easily digestible.
Immediately commission a third-party WCAG 2.1 AA audit and publish a formal 'Accessibility Statement' to mitigate significant legal risk and ensure the site is usable for all patients and clinicians.
The company's credibility is fundamentally anchored in its extensive, peer-reviewed clinical data, which is the gold standard for third-party validation in the medical field. Legal risk mitigation is also strong, with clear disclaimers. However, the on-site presentation of this credibility is weak, lacking easily digestible social proof like patient testimonials or key statistics, which forces users to dig for evidence of their success.
Credibility is fundamentally rooted in an unmatched portfolio of long-term, peer-reviewed clinical trial data (e.g., PARTNER trials), which serves as the ultimate third-party validation for its technology.
Integrate a prominent 'Evidence & Outcomes' section on the homepage featuring key statistics (e.g., '1M+ Patients Treated') and video testimonials to provide tangible, easily accessible proof of their impact.
Edwards possesses a formidable and highly sustainable competitive moat built on its first-mover advantage, a deep intellectual property portfolio, and decades of proprietary clinical data that is exceptionally difficult for competitors to replicate. This is reinforced by high switching costs for clinicians and hospitals trained on its ecosystem. The business model is sharply focused on structural heart, unlike more diversified competitors, allowing for dedicated innovation.
A highly sustainable competitive moat built on pioneering the TAVR market, extensive proprietary clinical data, and a deep intellectual property portfolio, creating high switching costs for clinicians.
Develop and integrate AI-powered pre-procedural planning tools for physicians to turn a potential technological threat into a competitive advantage, further embedding Edwards into the clinical workflow.
The company is exceptionally well-positioned for growth, with strong unit economics, high gross margins, and a massive war chest from a recent $4.2B divestiture. Major growth vectors are clearly defined in new therapies (TMTT), expanded indications for existing products, and geographic expansion into APAC. The primary constraint on scalability is operational: the ability to scale the highly specialized training required for physician adoption of new, complex procedures.
The business is exceptionally capitalized and has a clear, multi-pronged expansion strategy focused on new therapies (TMTT), new patient populations (lower-risk TAVR), and new geographies (APAC).
Invest heavily in scalable physician training technologies, such as VR/AR simulations and on-demand digital learning modules, to overcome the primary operational bottleneck limiting the pace of new therapy adoption.
The business model has exceptional coherence and strategic focus, particularly after the recent divestiture of its Critical Care unit to concentrate purely on the high-growth structural heart market. Resource allocation is precisely targeted at R&D and market expansion in its core area. The only minor weakness is a current over-reliance on the TAVR product line for revenue, a concentration risk the company is actively mitigating with its TMTT strategy.
Exceptional strategic focus, exemplified by the recent divestiture of its Critical Care unit, which allows for the precise allocation of capital and R&D resources to dominate the core structural heart market.
Aggressively accelerate the commercialization of the TMTT portfolio to build a second major growth engine, thereby mitigating the strategic risk of over-reliance on the maturing TAVR product line.
As the market pioneer and leader in TAVR with a dominant market share, Edwards wields significant market power. This position allows for premium pricing, influences clinical practice guidelines, and creates strong leverage with hospital partners. While competition from Medtronic and Abbott is intense, Edwards' deep well of long-term clinical data provides a powerful defense and reinforces its 'gold standard' reputation.
Dominant market leadership in the core TAVR segment, which provides significant pricing power and the ability to influence industry standards and clinical guidelines through its extensive body of evidence.
Proactively launch targeted digital campaigns for clinicians that directly address competitive clinical trial data (e.g., from Medtronic), emphasizing long-term durability and patient selection criteria to defend its market narrative.
Business Overview
Business Classification
B2B Medical Device Manufacturing
Healthcare Solutions & Clinical Support
Medical Technology (MedTech)
Sub Verticals
- •
Cardiovascular Devices
- •
Structural Heart Disease
- •
Transcatheter Heart Valves
- •
Surgical Heart Valves
Mature
Maturity Indicators
- •
Established in 1958, demonstrating a long and stable history.
- •
Dominant market share in key product categories, particularly Transcatheter Aortic Valve Replacement (TAVR).
- •
Recent strategic divestiture of its Critical Care division for $4.2 billion to focus on core high-growth structural heart business.
- •
Consistent revenue growth and significant investment in R&D (approx. 17-18% of sales).
- •
Global commercial presence with ~60% of sales from outside the US.
Enterprise
Steady Growth
Revenue Model
Primary Revenue Streams
- Stream Name:
Transcatheter Aortic Valve Replacement (TAVR) Product Sales
Description:Sales of the SAPIEN family of transcatheter heart valves and associated delivery systems. This is the company's largest and highest-performing revenue source, accounting for the majority of total sales.
Estimated Importance:Primary
Customer Segment:Interventional Cardiologists & Hospitals
Estimated Margin:High
- Stream Name:
Transcatheter Mitral and Tricuspid Therapies (TMTT) Product Sales
Description:Sales of devices for minimally invasive mitral and tricuspid valve repair and replacement, such as the PASCAL and EVOQUE systems. This is a key high-growth area for the company.
Estimated Importance:Secondary
Customer Segment:Interventional Cardiologists & Structural Heart Teams
Estimated Margin:High
- Stream Name:
Surgical Structural Heart Product Sales
Description:Sales of traditional surgical heart valves, including tissue valves and repair products like the PERIMOUNT and INSPIRIS RESILIA lines.
Estimated Importance:Tertiary
Customer Segment:Cardiac Surgeons & Hospitals
Estimated Margin:Medium
Recurring Revenue Components
- •
Procedure-driven repeat purchases of valves and devices.
- •
Service and support contracts for hospital equipment.
- •
Sales of disposable components used with capital systems.
Pricing Strategy
Value-Based Pricing
Premium
Opaque
Pricing Psychology
- •
Innovation Premium
- •
Clinical Superiority Justification
- •
Brand Trust & Reliability
Monetization Assessment
Strengths
- •
High gross profit margins (approx. 77-80%) driven by premium, innovative products.
- •
Dominant market position in the high-growth TAVR segment provides significant pricing power.
- •
Strong intellectual property portfolio creates a defensible market position.
Weaknesses
- •
High dependency on the TAVR market for a majority of revenue.
- •
Vulnerability to changes in healthcare reimbursement policies and hospital capital expenditure budgets.
- •
Complex sales cycle involving multiple stakeholders within hospital systems.
Opportunities
- •
Expansion of TAVR indications to lower-risk and asymptomatic patient populations, significantly increasing the total addressable market.
- •
Accelerated growth in the nascent, multi-billion dollar TMTT market.
- •
Geographic expansion in underpenetrated markets, especially in the Asia-Pacific region.
Threats
- •
Intensifying competition from large, well-funded medtech companies like Medtronic, Abbott, and Boston Scientific.
- •
Pricing pressure from hospital consolidation and government payers.
- •
Potential for disruptive technologies from smaller, innovative competitors.
Market Positioning
Innovation Leader & Clinical Evidence Champion
Market Leader (Controls approx. 60% of the worldwide TAVR market and over 70% in the U.S.).
Target Segments
- Segment Name:
Interventional Cardiologists & Cardiac Surgeons
Description:Specialized physicians who perform heart valve procedures. They are the primary users and key decision-makers for device selection.
Demographic Factors
Board-certified physicians
Affiliated with high-volume cardiovascular centers
Psychographic Factors
- •
Value clinical evidence and patient outcomes above all
- •
Seek innovative, reliable, and efficient technologies
- •
Reputation-conscious and influenced by peer-reviewed data and key opinion leaders
Behavioral Factors
- •
Attend major cardiology conferences (e.g., TCT)
- •
Participate in clinical trials and company-sponsored training
- •
Loyal to device platforms with which they have extensive training and successful experience
Pain Points
- •
Procedural complexity and risk of complications
- •
Need for durable, long-lasting valve solutions
- •
Time pressure in the catheterization lab/operating room
Fit Assessment:Excellent
Segment Potential:High
- Segment Name:
Hospital & Health System Administrators
Description:Executives (CEOs, CFOs, COOs) and value analysis committees responsible for procurement, budget allocation, and evaluating the economic value of new technologies.
Demographic Factors
Hold advanced degrees in business or healthcare administration
Manage large, complex budgets
Psychographic Factors
- •
Focused on cost-effectiveness and return on investment
- •
Value technologies that improve hospital reputation and patient throughput
- •
Risk-averse, preferring established vendors with strong support
Behavioral Factors
- •
Negotiate contracts through Group Purchasing Organizations (GPOs)
- •
Analyze total cost of care and reimbursement data
- •
Prioritize products that enable high-margin procedures and reduce length-of-stay
Pain Points
- •
Balancing high cost of innovation with budget constraints
- •
Navigating complex reimbursement landscapes
- •
Ensuring new technology adoption delivers measurable economic and clinical benefits
Fit Assessment:Good
Segment Potential:Medium
Market Differentiation
- Factor:
Pioneering Innovation in TAVR
Strength:Strong
Sustainability:Sustainable
- Factor:
Breadth of Clinical Evidence
Strength:Strong
Sustainability:Sustainable
- Factor:
Strong Clinician Relationships & Training Programs
Strength:Strong
Sustainability:Sustainable
- Factor:
Brand Reputation and Trust
Strength:Strong
Sustainability:Sustainable
Value Proposition
To be the global leader in patient-focused medical innovations for structural heart disease, improving and enhancing lives through partnerships with clinicians and stakeholders.
Excellent
Key Benefits
- Benefit:
Improved Patient Outcomes
Importance:Critical
Differentiation:Somewhat unique
Proof Elements
- •
Extensive portfolio of peer-reviewed clinical trial data
- •
Long-term patient follow-up studies
- •
Market leadership and high adoption rates
- Benefit:
Less Invasive Treatment Options
Importance:Critical
Differentiation:Unique
Proof Elements
Pioneering of the SAPIEN transcatheter heart valve platform
Ongoing development of next-generation TAVR, TMTT devices
- Benefit:
Comprehensive Clinician Support and Training
Importance:Important
Differentiation:Somewhat unique
Proof Elements
- •
Dedicated clinical education programs
- •
In-person and virtual proctoring
- •
Technical support for procedures
Unique Selling Points
- Usp:
Unmatched portfolio of long-term clinical evidence supporting the safety and efficacy of the SAPIEN TAVR platform.
Sustainability:Long-term
Defensibility:Strong
- Usp:
Pioneer and first-mover advantage in the transcatheter aortic valve market, building deep brand equity and trust.
Sustainability:Long-term
Defensibility:Strong
- Usp:
A focused, 'pure-play' structural heart company post-divestiture, enabling dedicated innovation and investment in the core market.
Sustainability:Medium-term
Defensibility:Moderate
Customer Problems Solved
- Problem:
Treating high-risk or inoperable patients with severe aortic stenosis.
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Reducing the invasiveness and recovery time associated with traditional open-heart valve surgery.
Severity:Major
Solution Effectiveness:Complete
- Problem:
Lack of effective, minimally invasive treatment options for complex mitral and tricuspid valve diseases.
Severity:Critical
Solution Effectiveness:Partial
Value Alignment Assessment
High
The value proposition directly addresses the market's shift towards minimally invasive procedures, driven by an aging population and the demand for better, safer treatments for structural heart disease.
High
The focus on clinical evidence, innovation, and improved outcomes aligns perfectly with the primary needs and values of cardiologists, surgeons, and hospital systems.
Strategic Assessment
Business Model Canvas
Key Partners
- •
Leading academic medical centers and research institutions
- •
Key Opinion Leaders (KOLs) in cardiology and cardiac surgery
- •
Regulatory bodies (e.g., FDA, EMA)
- •
Group Purchasing Organizations (GPOs) and hospital networks
- •
Specialized component suppliers
Key Activities
- •
Research & Development (R&D) for new devices and indications
- •
Conducting large-scale, global clinical trials
- •
High-precision, quality-controlled manufacturing
- •
Global sales and marketing to clinical specialists
- •
Physician training and education
Key Resources
- •
Extensive portfolio of patents and intellectual property
- •
World-class R&D and engineering talent
- •
Established global sales and clinical support infrastructure
- •
Strong brand reputation and clinical credibility
- •
Substantial clinical data and evidence
Cost Structure
- •
High R&D expenditures (17-19% of sales).
- •
Selling, General & Administrative (SG&A) expenses, including a large, specialized sales force.
- •
Cost of Goods Sold (COGS) for complex, high-value devices.
- •
Expenses for conducting extensive clinical trials.
Swot Analysis
Strengths
- •
Dominant market leadership in the core TAVR segment.
- •
Strong brand equity built on a foundation of clinical evidence and innovation.
- •
High profitability and strong cash flow generation.
- •
Sharpened strategic focus on high-growth structural heart markets following the divestiture of the Critical Care unit.
Weaknesses
- •
Over-reliance on the TAVR product line for revenue and growth.
- •
High barriers to adoption for new technologies, requiring extensive training and clinical data.
- •
Exposure to product recalls which can damage reputation and investor confidence.
Opportunities
- •
Tapping into the large, underserved market for Transcatheter Mitral and Tricuspid Therapies (TMTT), projected to reach $5 billion by 2028.
- •
Expanding TAVR indications into younger, lower-risk patient populations.
- •
Leveraging the $4.2B in proceeds from the Critical Care sale for strategic acquisitions and R&D in emerging areas.
Threats
- •
Intense competition from Medtronic, Abbott, and Boston Scientific, who possess significant resources.
- •
Increased pricing pressure from healthcare systems and GPOs.
- •
Stringent and evolving regulatory environments in key markets.
- •
Potential for disruptive technological advancements from competitors.
Recommendations
Priority Improvements
- Area:
Revenue Diversification
Recommendation:Aggressively accelerate the commercialization and clinical evidence generation for the TMTT portfolio (PASCAL, EVOQUE) to build a second major growth engine and reduce dependency on TAVR.
Expected Impact:High
- Area:
Market Penetration
Recommendation:Focus clinical trial and marketing efforts on expanding TAVR into the asymptomatic severe aortic stenosis patient population to unlock the next significant wave of market growth.
Expected Impact:High
- Area:
Operational Efficiency
Recommendation:Integrate digital health and AI-powered solutions for procedure planning and remote patient follow-up to create stickiness and add value beyond the device, enhancing the overall ecosystem.
Expected Impact:Medium
Business Model Innovation
Develop risk-sharing or outcomes-based pricing models with payers and providers, tying reimbursement to demonstrated patient outcomes to defend premium pricing and deepen partnerships.
Create a 'Structural Heart Center of Excellence' service model, offering a bundled solution of devices, training, data analytics, and patient management software to partner hospitals.
Revenue Diversification
- •
Continue strategic, disciplined M&A to acquire promising early-stage technologies in adjacent fields like interventional heart failure.
- •
Expand the surgical valve portfolio with next-generation RESILIA tissue technology to maintain leadership and capture share in the traditional surgery market.
- •
Invest in building commercial infrastructure in high-growth emerging markets to capitalize on the rising prevalence of cardiovascular disease.
Edwards Lifesciences has successfully executed a strategic evolution, transforming from a diversified medical device company into a focused, 'pure-play' powerhouse in structural heart disease. The recent $4.2 billion divestiture of its lower-margin Critical Care division is a pivotal move, sharpening its focus on the high-growth, high-margin TAVR and emerging TMTT markets. This positions the company to dedicate its substantial R&D investment (approaching 18% of sales) and commercial resources toward extending its market dominance.
The business model is built on a foundation of pioneering innovation, backed by an unparalleled volume of clinical evidence, which creates a strong competitive moat and justifies its premium pricing strategy. Its primary revenue driver, the TAVR segment, is a mature but still growing market, with significant expansion potential as indications broaden to include lower-risk patients. The key to Edwards' long-term trajectory is its ability to replicate its TAVR success in the nascent but potentially vast TMTT market. While its leadership is clear, the company faces intense competition from formidable, larger rivals and must continuously innovate to protect its market share and premium positioning. The strategic challenge is to balance investment in the core TAVR franchise while successfully scaling the TMTT business to become the next major pillar of growth, thereby diversifying its revenue base and securing its leadership for the next decade.
Competitors
Competitive Landscape
Mature
Oligopoly
Barriers To Entry
- Barrier:
Stringent Regulatory Approval (e.g., FDA, CE Mark)
Impact:High
- Barrier:
Extensive & Costly Clinical Trials
Impact:High
- Barrier:
Intellectual Property & Patent Protection
Impact:High
- Barrier:
High R&D Investment Requirements
Impact:High
- Barrier:
Established Physician/Hospital Relationships & Brand Trust
Impact:Medium
- Barrier:
Complex Manufacturing & Quality Control
Impact:Medium
Industry Trends
- Trend:
Shift Towards Minimally Invasive Procedures (TAVR/TMVR)
Impact On Business:Positive. This is Edwards' core business and a primary growth driver as patient eligibility expands to lower-risk populations.
Timeline:Immediate
- Trend:
Integration of AI for Diagnosis & Procedural Planning
Impact On Business:Opportunity. AI can enhance patient selection, device sizing, and procedural outcomes, creating a new axis of competition.
Timeline:Near-term
- Trend:
Focus on Lifetime Management of Heart Valve Disease
Impact On Business:Critical. Requires focus on long-term device durability (e.g., RESILIA tissue technology) and developing solutions for younger patients.
Timeline:Immediate
- Trend:
Expansion into Tricuspid & Mitral Valve Therapies
Impact On Business:High Growth Opportunity. These are less mature markets than aortic valves, representing a significant avenue for expansion and market leadership.
Timeline:Near-term
- Trend:
Value-Based Healthcare & Economic Outcomes
Impact On Business:Requires demonstration of not just clinical efficacy but also cost-effectiveness (e.g., reduced hospital stays) to secure reimbursement and hospital contracts.
Timeline:Immediate
Direct Competitors
- →
Medtronic
Market Share Estimate:Significant, #2 player in TAVR market
Target Audience Overlap:High
Competitive Positioning:Broad-portfolio med-tech giant with a strong focus on self-expanding TAVR valves.
Strengths
- •
Strong clinical data showing superior hemodynamics for its Evolut valve in small annuli patients.
- •
Extensive global sales and distribution network across multiple hospital departments.
- •
Aggressive R&D and clinical trial strategy to expand indications.
- •
Self-expanding valve design (Evolut platform) is a key differentiator, particularly effective in certain anatomies.
Weaknesses
- •
Historically trailed Edwards in the TAVR market, playing catch-up on brand leadership.
- •
Portfolio is less focused on structural heart compared to Edwards, potentially diluting strategic attention.
- •
Higher rates of pacemaker implantation have been a concern with earlier generation self-expanding valves.
Differentiators
- •
Supra-annular, self-expanding valve design of the Evolut platform.
- •
Focus on generating head-to-head competitive clinical data (e.g., SMART trial).
- •
Broad portfolio of other cardiovascular devices, allowing for potential bundling.
- →
Abbott Laboratories
Market Share Estimate:Leader in mitral repair, growing player in other areas
Target Audience Overlap:High
Competitive Positioning:Leader in transcatheter mitral and tricuspid repair with a growing portfolio of replacement and aortic valve technologies.
Strengths
- •
Dominant market leader in transcatheter mitral valve repair with MitraClip.
- •
Broadest structural heart portfolio, covering repair and replacement across multiple valves (MitraClip, TriClip, Tendyne, Navitor).
- •
Strong brand recognition and established presence in cath labs.
- •
First-mover advantage in the transcatheter tricuspid repair market (TriClip).
Weaknesses
- •
Later entrant into the TAVR market, significantly behind Edwards and Medtronic in this specific segment.
- •
Manages a highly diversified healthcare business, which could impact focus on structural heart.
- •
Tendyne (mitral replacement) addresses a more niche, high-risk patient population currently.
Differentiators
- •
Pioneering and leading the transcatheter edge-to-edge repair (TEER) market segment.
- •
Comprehensive portfolio strategy aiming to provide a solution for nearly every structural heart defect.
- •
Strong focus on clinical evidence to expand indications for its repair devices.
- →
Boston Scientific
Market Share Estimate:Minimal in TAVR after product discontinuation
Target Audience Overlap:High
Competitive Positioning:Major interventional cardiology player, but currently weakened in the TAVR space after failing to gain market traction.
Strengths
- •
Very strong presence in other areas of interventional cardiology (e.g., coronary stents, WATCHMAN LAA closure device).
- •
Deep, established relationships with interventional cardiologists.
- •
Strong global sales and marketing infrastructure.
Weaknesses
- •
Discontinued its ACURATE neo2 TAVR valve program in the U.S. and other markets after unfavorable clinical trial results compared to Edwards and Medtronic.
- •
Significant gap in their structural heart portfolio without a competitive TAVR offering.
- •
Loss of credibility in the TAVR space, making re-entry difficult and costly.
Differentiators
Market leadership in Left Atrial Appendage (LAA) closure with the WATCHMAN device, a complementary structural heart procedure.
Focus on other high-growth cardiology segments.
Indirect Competitors
- →
Pharmaceutical Companies
Description:Companies developing drugs to slow or reverse the progression of aortic stenosis or other valvular diseases, potentially reducing the future need for valve replacement.
Threat Level:Low
Potential For Direct Competition:Low in the near-term, but a breakthrough drug could be a long-term market disruptor.
- →
Surgical Robotics Companies (e.g., Intuitive Surgical)
Description:Develop robotic systems that assist in minimally invasive surgeries. While often a partner, they could develop specialized instruments that compete with transcatheter approaches.
Threat Level:Low
Potential For Direct Competition:Low, as their platforms are typically used for surgical valve replacement, which is a different procedure. They are more of an enabler for surgeons than a direct competitor to TAVR devices.
- →
Early-Stage MedTech Startups
Description:Startups developing novel valve technologies, such as polymer-based valves for enhanced durability, 3D-printed custom valves, or regenerative tissue-engineered valves.
Threat Level:Medium
Potential For Direct Competition:High, as they are often acquisition targets for major players or can introduce disruptive technology.
Competitive Advantage Analysis
Sustainable Advantages
- Advantage:
Market Leadership & Brand Reputation in TAVR
Sustainability Assessment:Highly sustainable due to decades of clinical data, physician trust, and procedural familiarity with the SAPIEN valve platform.
Competitor Replication Difficulty:Hard
- Advantage:
Extensive Proprietary Clinical Data
Sustainability Assessment:Highly sustainable. Long-term data from pivotal trials like PARTNER are extremely difficult and time-consuming for competitors to replicate.
Competitor Replication Difficulty:Hard
- Advantage:
Intellectual Property Portfolio
Sustainability Assessment:Sustainable. A deep patent portfolio around balloon-expandable valve technology and delivery systems creates significant barriers.
Competitor Replication Difficulty:Hard
- Advantage:
Focused Business Model
Sustainability Assessment:Moderately sustainable. The recent sale of the Critical Care business allows for a laser focus on the high-growth structural heart market, unlike more diversified competitors.
Competitor Replication Difficulty:Medium
Temporary Advantages
{'advantage': 'First-Mover in New Indications', 'estimated_duration': '1-3 years. Being the first to gain approval for new patient populations (e.g., asymptomatic AS) provides a significant but temporary head start before competitors complete their own trials.'}
{'advantage': 'Specific Technological Leads (e.g., RESILIA tissue)', 'estimated_duration': '3-5 years. Innovations in tissue technology provide a durability advantage, but competitors are actively researching next-generation materials.'}
Disadvantages
- Disadvantage:
Premium Pricing Strategy
Impact:Major
Addressability:Difficult. The premium price is justified by clinical data, but faces pressure from competitors and value-based purchasing models in healthcare systems.
- Disadvantage:
Less Diversified Portfolio Post-Divestiture
Impact:Minor
Addressability:Moderately. While it allows for focus, it also increases reliance on the structural heart market and reduces cross-selling opportunities compared to Medtronic or Abbott.
- Disadvantage:
Perceived Hemodynamic Disadvantage in Small Annuli
Impact:Major
Addressability:Difficult. Medtronic's SMART trial data has created a strong competitive narrative that Edwards must counter with its own data and marketing.
Strategic Recommendations
Quick Wins
- Recommendation:
Launch a targeted digital campaign for clinicians directly addressing the Medtronic SMART trial data, emphasizing patient selection criteria and long-term durability outcomes from the PARTNER trials.
Expected Impact:Medium
Implementation Difficulty:Easy
- Recommendation:
Amplify marketing of the SAPIEN M3 and EVOQUE approvals to highlight the completeness of the TMTT portfolio, positioning Edwards as the leader in both mitral and tricuspid solutions.
Expected Impact:High
Implementation Difficulty:Moderate
Medium Term Strategies
- Recommendation:
Invest heavily in generating real-world evidence and new clinical trials focusing on the long-term (10+ year) durability of the SAPIEN platform with RESILIA tissue to counter competitor claims and solidify the 'lifetime management' narrative.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Develop and integrate AI-powered pre-procedural planning tools for physicians to optimize valve sizing and positioning, turning a potential threat into a competitive advantage.
Expected Impact:Medium
Implementation Difficulty:Moderate
Long Term Strategies
- Recommendation:
Acquire or partner with startups in the polymer or regenerative valve space to secure a next-generation technology pipeline beyond bioprosthetic tissue.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Expand into digital health solutions for post-TAVR patient monitoring to own the entire patient journey and generate valuable long-term outcome data.
Expected Impact:Medium
Implementation Difficulty:Moderate
Reinforce and aggressively defend the position as the 'Gold Standard Innovator' in structural heart, emphasizing unmatched long-term clinical evidence, procedural predictability, and superior device durability.
Differentiate on the basis of a complete 'lifetime management' solution, from the industry's most trusted TAVR platform to a comprehensive and leading portfolio for mitral and tricuspid therapies.
Whitespace Opportunities
- Opportunity:
Develop a dedicated TAVR platform specifically engineered and marketed for younger, lower-risk patients with a 20+ year life expectancy.
Competitive Gap:All current devices are primarily focused on older populations; a valve with proven superior durability and minimal need for anticoagulation would dominate this emerging segment.
Feasibility:Medium
Potential Impact:High
- Opportunity:
Create an integrated digital ecosystem for Heart Teams, combining diagnostics, AI-driven procedural planning, and long-term patient outcome tracking.
Competitive Gap:Competitors offer devices, but no single company provides a comprehensive software and data platform that streamlines the entire patient care pathway.
Feasibility:Medium
Potential Impact:High
- Opportunity:
Pioneer the treatment of moderate, symptomatic aortic stenosis.
Competitive Gap:Current guidelines focus on severe AS. Successfully expanding the indication to moderate AS through strong clinical trial data (like the EARLY TAVR trial) would dramatically increase the total addressable market.
Feasibility:High
Potential Impact:High
Edwards Lifesciences operates in the mature but high-growth structural heart device market, which is best characterized as an oligopoly. The company enjoys a commanding leadership position in the Transcatheter Aortic Valve Replacement (TAVR) market, built on a foundation of pioneering technology (SAPIEN platform), extensive long-term clinical data, and deep-rooted trust within the clinical community. This position is protected by high barriers to entry, including rigorous regulatory pathways, intellectual property, and the immense cost of clinical validation.
The competitive landscape is intense and dominated by three key players. Medtronic stands as the primary challenger in the TAVR space, leveraging its self-expanding Evolut platform to create a strong competitive narrative, particularly in patients with small aortic annuli. Abbott Laboratories dominates the transcatheter mitral and tricuspid repair markets with its MitraClip and TriClip devices and is methodically building a comprehensive portfolio to challenge Edwards across all valves. Boston Scientific has effectively exited the TAVR race for now, ceding market share but remaining a formidable competitor in adjacent structural heart segments like LAA closure.
Edwards' core sustainable advantages are its brand equity as the TAVR pioneer and its unparalleled body of long-term clinical evidence, which is incredibly difficult for competitors to replicate. The recent divestiture of its Critical Care business sharpens its focus on the structural heart market, a key strategic advantage over its more diversified rivals. However, the company faces competitive disadvantages, including a premium pricing strategy that is susceptible to pressure and a direct clinical challenge from Medtronic's recent trial data.
Strategic imperatives for Edwards must focus on three pillars: 1) Defend its TAVR leadership by aggressively marketing its long-term durability data and innovating the SAPIEN platform. 2) Accelerate growth and establish leadership in the burgeoning Transcatheter Mitral and Tricuspid Therapies (TMTT) market, leveraging its new comprehensive portfolio. 3) Invest in next-generation technologies (e.g., polymer valves, AI planning tools) to preempt market disruption and address the needs of younger patients, thereby securing its leadership for the next decade.
Messaging
Message Architecture
Key Messages
- Message:
Edwards Lifesciences is driven by a passion to help patients.
Prominence:Primary
Clarity Score:High
Location:Main Homepage Headline
- Message:
We are dedicated to providing innovative solutions for people fighting cardiovascular disease.
Prominence:Secondary
Clarity Score:High
Location:Patients Section
- Message:
Clinical research means new hope for others.
Prominence:Tertiary
Clarity Score:High
Location:Clinical Research Information Section
- Message:
Innovation starts with human inspiration.
Prominence:Tertiary
Clarity Score:Medium
Location:Careers Section
The message hierarchy is logical and effective. It correctly prioritizes the core brand driver ('passion for patients') at the highest level. This central theme is then supported by messages of innovation, hope through research, and corporate responsibility. The structure successfully communicates who they are and what they value before directing users to specific product or audience pathways.
Messaging is exceptionally consistent across the homepage. The core tenets of patient-centricity, passion, and innovation are woven into nearly every section, from the main headline to the careers CTA. This repetition reinforces the brand's purpose and creates a unified, memorable impression.
Brand Voice
Voice Attributes
- Attribute:
Passionate
Strength:Strong
Examples
driven by a passion to help patients
Innovation starts with human inspiration
- Attribute:
Patient-Centric
Strength:Strong
Examples
Our patient-focused innovative technologies
We are dedicated to providing innovative solutions for people fighting cardiovascular disease
- Attribute:
Innovative
Strength:Strong
Examples
- •
Our patient-focused innovative technologies
- •
providing innovative solutions
- •
Innovation starts with human inspiration
- Attribute:
Hopeful
Strength:Moderate
Examples
Clinical research means new hope for others
Tone Analysis
Purpose-driven
Secondary Tones
- •
Compassionate
- •
Professional
- •
Optimistic
Tone Shifts
The tone shifts to be more direct and empowering in the 'Aortic stenosis' section with the imperative 'Take control...'
The tone becomes more inspirational and employee-focused in the 'Careers' section with 'Come to be inspired.'
Voice Consistency Rating
Excellent
Consistency Issues
No itemsValue Proposition Assessment
Edwards Lifesciences is a mission-driven leader that leverages its passion for patients to create breakthrough innovations for structural heart disease, in partnership with clinicians.
Value Proposition Components
- Component:
Patient-Focused Innovation
Clarity:Clear
Uniqueness:Somewhat Unique
- Component:
Leadership in Structural Heart Disease
Clarity:Somewhat Clear
Uniqueness:Unique
- Component:
Hope through Clinical Research
Clarity:Clear
Uniqueness:Common
- Component:
Partnership with Clinicians
Clarity:Somewhat Clear
Uniqueness:Common
The primary differentiator is the emotional framing of its mission through the concept of 'passion'. While competitors like Medtronic and Boston Scientific also focus on innovation and patient outcomes, Edwards' language is more emotive and purpose-driven. This creates a powerful brand identity but could be strengthened by more explicitly stating its leadership and sharpened focus on structural heart disease following the sale of its Critical Care division.
The messaging positions Edwards as the conscientious, passionate leader in the field. It aims to build brand preference not just on product superiority (which is implied) but on a shared mission and value system. This is a strong position to take in an industry where trust and purpose are paramount, appealing to both clinicians and patients on an emotional level.
Audience Messaging
Target Personas
- Persona:
Patients & Caregivers
Tailored Messages
- •
driven by a passion to help patients
- •
We are dedicated to providing innovative solutions for people fighting cardiovascular disease
- •
Take control of your severe aortic stenosis
Effectiveness:Effective
- Persona:
Healthcare Professionals (Clinicians, Surgeons)
Tailored Messages
- •
Our patient-focused innovative technologies
- •
Transcatheter heart valves
- •
Clinical research information
Effectiveness:Somewhat Effective
- Persona:
Potential Employees
Tailored Messages
Innovation starts with human inspiration
Come to be inspired
Effectiveness:Effective
Audience Pain Points Addressed
- •
Fighting cardiovascular disease
- •
Living with severe aortic stenosis
- •
The need for less invasive treatment options (implied by 'Transcatheter' technologies)
Audience Aspirations Addressed
- •
A desire for hope and new solutions
- •
The goal of improving and enhancing lives
- •
Aspiration to regain control over one's health
Persuasion Elements
Emotional Appeals
- Appeal Type:
Pathos (Empathy and Passion)
Effectiveness:High
Examples
driven by a passion to help patients
- Appeal Type:
Hope
Effectiveness:High
Examples
Clinical research means new hope for others
Social Proof Elements
No itemsTrust Indicators
Mention of 'Clinical research' implies scientific rigor.
Highlighting 'Corporate Impact' and 'Global corporate giving' builds an image of an ethical, responsible company.
Scarcity Urgency Tactics
No itemsCalls To Action
Primary Ctas
- Text:
More about us
Location:Patients Section
Clarity:Clear
- Text:
Patients and Care Partners
Location:Clinical Research Section
Clarity:Clear
- Text:
Healthcare Professionals
Location:Clinical Research Section
Clarity:Clear
- Text:
Visit treatheartvalvefailure.com
Location:Aortic Stenosis Section
Clarity:Clear
- Text:
Come to be inspired
Location:Careers Section
Clarity:Clear
The CTAs are clear and effectively segment the audience, directing them to relevant information silos. However, their format as simple hyperlinks rather than visually prominent buttons may reduce their click-through rate. The actions are primarily informational ('learn,' 'visit'), which is appropriate for a corporate homepage but lacks direct lead-generation or engagement-driving actions.
Messaging Gaps Analysis
Critical Gaps
- •
Lack of Social Proof: The homepage makes powerful emotional claims ('passion,' 'dedication') but provides no evidence to back them up, such as patient stories, clinician testimonials, or key statistics (e.g., 'X million lives improved').
- •
Absence of Leadership Assertion: While their corporate mission states they are the 'global leader', this powerful positioning is entirely missing from the homepage messaging, ceding a key competitive advantage.
- •
Vague Clinician Value Proposition: The site doesn't articulate why a clinician should partner with Edwards over a competitor. Benefits like superior clinical support, training, or patient outcomes data are not mentioned.
- •
Missing Clinical Evidence: Beyond the mention of 'research', there is no top-level data, study results, or proof of efficacy to build credibility with a scientifically-minded clinician audience.
Contradiction Points
The 'Critical care technologies' product listing, which has been sold to BD, creates a confusing message about the company's current focus. The asterisk and small note are insufficient to clarify this significant strategic shift, potentially diluting their core message for new visitors.
Underdeveloped Areas
The messaging to healthcare professionals is underdeveloped. It consists of a list of product categories but lacks a narrative about how these technologies empower clinicians and improve their practice.
Messaging Quality
Strengths
- •
Extraordinarily clear and consistent patient-centric purpose.
- •
Strong, positive, and memorable brand voice rooted in 'passion'.
- •
Effective use of emotional appeal to create a connection with the audience.
- •
Excellent audience segmentation in navigation, particularly for Patients vs. Healthcare Professionals.
Weaknesses
- •
Over-reliance on abstract claims without concrete proof points.
- •
Failure to leverage and communicate established market leadership.
- •
Weak visual hierarchy for calls-to-action (links vs. buttons).
- •
Confusing messaging around a divested business unit.
Opportunities
- •
Feature a compelling patient or clinician story on the homepage to bring the 'passion' to life.
- •
Quantify their impact (e.g., 'Over 1 million patients helped') to add substance to their leadership claims.
- •
Sharpen the corporate narrative to focus on their market-leading position in structural heart disease post-divestiture.
- •
Develop specific messaging that details the tangible benefits of a partnership with Edwards for clinicians and hospitals.
Optimization Roadmap
Priority Improvements
- Area:
Social Proof & Trust
Recommendation:Integrate a 'Stories of Impact' section on the homepage featuring a rotating, high-quality patient or clinician video testimonial. This directly substantiates the 'passion for patients' claim.
Expected Impact:High
- Area:
Market Positioning
Recommendation:Revise the main headline or add a sub-headline to read: 'The global leader in structural heart innovation, driven by a passion to help patients.' This immediately claims their market position.
Expected Impact:High
- Area:
Message Clarity
Recommendation:Remove the 'Critical Care technologies' link from the main product list to reflect the company's current strategic focus. This eliminates audience confusion and sharpens the brand's narrative.
Expected Impact:Medium
Quick Wins
Convert the most important text-link CTAs (e.g., 'More about us,' 'Patients and Care Partners') into visually distinct buttons.
Add a statistics bar below the main header with key proof points like '65+ Years of Innovation', '1M+ Patients Impacted', '17-18% of Revenue Reinvested in R&D'.
Long Term Recommendations
- •
Develop a dedicated content hub for healthcare professionals that goes beyond products to include clinical data, case studies, and thought leadership on the future of structural heart treatment.
- •
Build a narrative around the 'partnership with clinicians,' showcasing how Edwards' support, data, and innovation lead to better outcomes and empower medical professionals.
- •
Create a messaging framework that clearly delineates the value proposition for different clinical sub-specialties (e.g., interventional cardiology vs. cardiac surgery).
Edwards Lifesciences has crafted a powerful, emotionally resonant, and highly consistent core message centered on a 'passion to help patients.' This patient-centricity serves as an excellent foundation for brand differentiation in the competitive medical technology space. The brand voice is strong, purpose-driven, and effectively communicates a sense of mission that appeals to patients, clinicians, and potential employees alike. The website's messaging architecture is clean, with a logical hierarchy and clear segmentation for its primary audiences.
However, the strategy's primary weakness is its over-reliance on this emotional appeal without providing the necessary substantiation. The homepage makes compelling claims but offers no social proof (patient stories, clinician testimonials) or logical proof (clinical data, impact statistics) to convert brand affinity into deep-seated trust and credibility. Key business strengths, such as their documented position as a 'global leader', are conspicuously absent from the messaging, representing a significant missed opportunity to assert market dominance. Furthermore, the inclusion of a recently divested business unit on the main product list creates unnecessary confusion, diluting their otherwise sharp focus on structural heart disease.
To elevate its messaging from good to great, Edwards must move from simply stating its passion to demonstrating its impact. By weaving in powerful stories, quantifying its achievements, and sharpening its positioning as the focused leader in its category, Edwards can build upon its strong emotional foundation to create an unassailable brand narrative that drives measurable business outcomes.
Growth Readiness
Growth Foundation
Product Market Fit
Strong
Evidence
- •
Dominant market share in the Transcatheter Aortic Valve Replacement (TAVR) market, controlling approximately 72.3% in the U.S. as of 2025.
- •
The SAPIEN platform is widely considered an industry leader with strong clinical data and high adoption rates by top cardiologists and hospitals globally.
- •
Pioneer and first-mover advantage in the U.S. TAVR market since 2011, establishing a deep moat of clinical evidence and physician relationships.
- •
Consistently high R&D investment (17-18% of sales) focused on next-generation structural heart innovations, demonstrating a commitment to solving unmet clinical needs.
Improvement Areas
- •
Accelerate adoption and positive clinical outcomes for the newer Transcatheter Mitral and Tricuspid Therapies (TMTT) portfolio to replicate TAVR's success.
- •
Strengthen the value proposition for expanding TAVR into lower-risk and asymptomatic patient populations to continue market growth.
- •
Enhance direct-to-patient educational initiatives to increase awareness of aortic stenosis and drive patient-led demand for evaluation and treatment.
Market Dynamics
The global structural heart devices market is projected to grow at a strong CAGR, with estimates ranging from 8.0% to 13.5% annually.
Growing
Market Trends
- Trend:
Shift towards minimally invasive procedures
Business Impact:This is a significant tailwind for Edwards' core business in transcatheter valves (TAVR, TMTT), driving adoption over traditional open-heart surgery.
- Trend:
Aging global population
Business Impact:Increases the prevalence of cardiovascular and valvular heart diseases, directly expanding the addressable patient population for Edwards' products.
- Trend:
Focus on value-based healthcare
Business Impact:Requires robust clinical and economic evidence to secure reimbursement and hospital contracts, an area where Edwards has historically excelled.
- Trend:
Increased competition in the TAVR space
Business Impact:New entrants like Abbott and a re-emerging Boston Scientific are expected to cause minor market share erosion, necessitating continuous innovation.
Excellent. The recent divestiture of the Critical Care business for $4.2 billion provides capital and 'laser focus' on the high-growth structural heart market, enabling accelerated investment in core growth areas like TMTT.
Business Model Scalability
High
High fixed costs in R&D and clinical trials, but highly scalable manufacturing and sales model with strong gross margins once products are commercialized.
High. Each incremental sale of a high-margin device contributes significantly to profitability after covering initial R&D and market access costs.
Scalability Constraints
- •
Scaling the highly specialized direct sales and clinical support teams required to train physicians.
- •
Manufacturing capacity for complex, high-precision medical devices.
- •
Navigating country-by-country regulatory approval processes for new products and indications.
Team Readiness
Proven and experienced leadership team with a clear strategic focus on structural heart disease following the Critical Care divestiture.
Well-suited for innovation and commercial execution, with dedicated business units for TAVR, TMTT, and Surgical, allowing for focused strategies.
Key Capability Gaps
- •
Expanding digital health and data science capabilities to integrate AI-driven procedural planning and post-operative monitoring tools.
- •
Building out market access and reimbursement expertise specifically for the nascent TMTT market in new geographies.
- •
Developing more sophisticated direct-to-patient marketing and engagement skills to build disease awareness.
Growth Engine
Acquisition Channels
- Channel:
Direct Sales & Clinical Specialists
Effectiveness:High
Optimization Potential:Medium
Recommendation:Equip the sales force with advanced data analytics tools to identify high-potential hospitals and surgeons; implement hybrid sales models combining in-person support with remote education.
- Channel:
Medical Conferences & Publications
Effectiveness:High
Optimization Potential:Low
Recommendation:Maintain leadership presence and continue to present landmark clinical trial data, which is the primary driver of adoption in this industry.
- Channel:
Physician Training & Education Programs
Effectiveness:High
Optimization Potential:High
Recommendation:Invest in scalable, next-generation training platforms (e.g., VR/AR simulation) to accelerate the learning curve for new physicians and support staff, reducing a key adoption bottleneck.
Customer Journey
The 'customer' is the clinician/hospital. The path is complex: Awareness (clinical data) -> Education (training) -> Validation (peer-to-peer advocacy) -> Procurement (hospital value analysis committee) -> Adoption (first case) -> Loyalty (ongoing support/next-gen products).
Friction Points
- •
Complex hospital procurement and value analysis committee approval processes.
- •
Significant training investment required for physicians and cath lab staff to adopt new procedures.
- •
Navigating reimbursement and payer coverage for new therapies and indications.
Journey Enhancement Priorities
{'area': 'Value Analysis Committee Engagement', 'recommendation': 'Develop tailored economic value propositions and ROI calculators for hospital administrators, complementing the clinical data provided to physicians.'}
{'area': 'Early Physician Training', 'recommendation': 'Create on-demand digital training modules to supplement in-person proctoring, making it easier for busy clinicians to begin the learning process.'}
Retention Mechanisms
- Mechanism:
Next-Generation Product Pipeline
Effectiveness:High
Improvement Opportunity:Ensure a predictable cadence of meaningful product enhancements (e.g., lower profile, improved durability) to incentivize loyalty and prevent competitor inroads.
- Mechanism:
On-site Clinical Support
Effectiveness:High
Improvement Opportunity:Leverage data from supported cases to provide proactive, predictive insights to partner hospitals on improving patient outcomes and efficiency.
- Mechanism:
Investigator-Initiated Research & Partnerships
Effectiveness:Medium
Improvement Opportunity:Establish a more streamlined program for partnering with leading academic centers on research, solidifying Edwards' position as a scientific partner, not just a vendor.
Revenue Economics
Very Strong. High-margin, differentiated medical devices with significant pricing power driven by strong intellectual property and extensive clinical data.
Qualitatively High. The 'CAC' includes massive R&D and clinical trial investments, but the 'LTV' of a hospital that adopts an Edwards valve platform is extremely high due to stickiness, repeat procedures, and adoption of future products.
High. The company demonstrates strong operating margins, which are expected to improve further after the divestiture of the lower-margin Critical Care business.
Optimization Recommendations
- •
Improve manufacturing efficiencies to protect gross margins against inflation and supply chain pressures.
- •
Develop tiered product offerings to address different price points in emerging markets without cannibalizing premium markets.
- •
Secure broader and more favorable reimbursement coverage for TMTT to accelerate revenue growth in that segment.
Scale Barriers
Technical Limitations
- Limitation:
Clinical Trial Risk
Impact:High
Solution Approach:Run a portfolio of clinical trials across different products and indications to diversify risk. Utilize adaptive trial designs to increase efficiency and probability of success.
- Limitation:
Long-Term Device Durability
Impact:Medium
Solution Approach:Continue investing in innovative tissue technologies like RESILIA to extend valve durability, a key factor for expanding into younger, lower-risk patient populations.
Operational Bottlenecks
- Bottleneck:
Physician Training Scalability
Growth Impact:Limits the pace of new therapy adoption, especially for complex procedures like mitral and tricuspid valve replacement.
Resolution Strategy:Invest heavily in scalable training technologies and 'train the trainer' programs at major medical centers to decentralize and accelerate education.
- Bottleneck:
Global Regulatory Approvals
Growth Impact:Each new product or indication faces a multi-year, country-specific approval process, delaying revenue generation.
Resolution Strategy:Build deep, in-country regulatory affairs teams and engage with regulators early in the development process to align on evidence requirements and streamline submissions.
- Bottleneck:
Sterile Manufacturing & Supply Chain
Growth Impact:Complex manufacturing processes for Class III medical devices can be difficult to scale and are vulnerable to supply chain disruptions.
Resolution Strategy:Increase vertical integration for critical components, qualify secondary suppliers, and invest in manufacturing automation to improve yield and capacity.
Market Penetration Challenges
- Challenge:
Intensifying Competition
Severity:Major
Mitigation Strategy:Maintain a high pace of innovation to ensure technology leadership. Leverage the extensive body of clinical evidence to defend market share and justify premium pricing. Compete on total value, including clinical support and outcomes, not just on device features.
- Challenge:
Reimbursement and Market Access
Severity:Critical
Mitigation Strategy:Generate robust health economics and outcomes research (HEOR) data to prove cost-effectiveness to payers. Partner with medical societies to establish favorable treatment guidelines that support new therapies.
- Challenge:
Patient Identification & Referral
Severity:Major
Mitigation Strategy:Invest in disease awareness campaigns and screening programs to identify untreated patients. Develop digital tools to help streamline the referral pathway from primary care and general cardiology to structural heart specialists.
Resource Limitations
Talent Gaps
- •
R&D engineers with expertise in AI, sensor technology, and data analytics.
- •
Regulatory affairs specialists with experience in emerging markets like China and India.
- •
Health economists and market access professionals with expertise in securing reimbursement for novel therapies.
Low. The company is well-capitalized, especially following the $4.2B cash sale of its Critical Care unit. Capital will be strategically deployed for R&D, tuck-in acquisitions, and share repurchases.
Infrastructure Needs
- •
Expansion of manufacturing facilities to meet projected demand for TAVR and TMTT.
- •
Investment in a global, scalable digital infrastructure for training and remote clinical support.
- •
Establishment of Centers of Excellence in key international markets to drive clinical adoption.
Growth Opportunities
Market Expansion
- Expansion Vector:
Geographic: Asia-Pacific (esp. China, India)
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Establish local partnerships for manufacturing and distribution. Invest in clinical trials specific to local populations to secure regulatory approval and physician buy-in, as market growth in APAC is expected to be the fastest.
- Expansion Vector:
Indication Expansion: TAVR for Lower-Risk & Asymptomatic Patients
Potential Impact:High
Implementation Complexity:Medium
Recommended Approach:Successfully complete and publish data from pivotal trials like EARLY TAVR to change clinical guidelines and unlock a significantly larger patient population.
- Expansion Vector:
New Disease States: Interventional Heart Failure
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Leverage the cash from the Critical Care sale to invest in R&D and strategic acquisitions for implantable heart failure technologies, a stated new area of focus.
Product Opportunities
- Opportunity:
Transcatheter Mitral & Tricuspid Therapies (TMTT)
Market Demand Evidence:Massive, underserved patient population. The global TMTT market is projected to reach $5 billion by 2028, with very low current penetration.
Strategic Fit:Perfect. This is the company's #1 stated growth priority and leverages existing expertise in transcatheter technology, clinical trials, and cardiologist relationships.
Development Recommendation:Aggressively fund the portfolio of differentiated TMTT products (e.g., PASCAL, EVOQUE, SAPIEN M3), execute pivotal trials flawlessly, and build the commercial infrastructure to support a major product launch cycle.
- Opportunity:
Digital Health & AI Integration
Market Demand Evidence:Clinicians are seeking tools to improve procedural precision, predictability, and efficiency.
Strategic Fit:High. Adds a high-margin, sticky software layer to the existing device business, enhancing the overall value proposition.
Development Recommendation:Acquire or partner with leading AI-based surgical planning and imaging analysis companies. Develop a platform that integrates pre-operative planning, intra-operative guidance, and post-operative monitoring.
Channel Diversification
- Channel:
Direct-to-Patient (DTP) Education Platforms
Fit Assessment:High. Empowers patients to seek care, especially for under-diagnosed conditions like aortic stenosis. Aligns with patient-focused mission.
Implementation Strategy:Expand websites like 'treatheartvalvefailure.com' with more interactive tools, patient testimonials, and physician finders. Launch targeted social media campaigns aimed at the geriatric demographic and their caregivers.
- Channel:
Ambulatory Surgical Centers (ASCs)
Fit Assessment:Medium (Future). As procedures become more routine and reimbursement shifts, some TAVR cases may move to lower-cost outpatient settings.
Implementation Strategy:Begin pilot programs with leading ASCs to develop the protocols, training, and economic models necessary for a potential future shift in site-of-care.
Strategic Partnerships
- Partnership Type:
Tuck-in Technology Acquisition
Potential Partners
- •
Startups with novel valve technologies (e.g., polymer-based valves)
- •
Companies with unique catheter or delivery system designs
- •
AI-powered cardiac imaging analysis firms
Expected Benefits:Accelerate R&D, acquire talent, and fill portfolio gaps in next-generation structural heart technologies.
- Partnership Type:
Hospital System Integration
Potential Partners
Major integrated delivery networks (IDNs)
Large hospital chains (e.g., HCA, Ascension)
Expected Benefits:Create long-term strategic partnerships focused on developing 'Structural Heart Centers of Excellence,' streamlining patient pathways, and standardizing on Edwards' technology platform.
Growth Strategy
North Star Metric
Number of Patients Treated Annually
This metric directly aligns with the company's mission to save and enhance lives. It holistically captures growth across all key vectors: new product adoption (TMTT), indication expansion (TAVR), and geographic penetration. It is a true measure of market impact.
12-15% year-over-year growth in patients treated, driven by an acceleration in TMTT adoption.
Growth Model
Medical Innovation & Clinical Adoption-Led Growth
Key Drivers
- •
Groundbreaking clinical evidence from pivotal trials.
- •
A highly skilled direct sales force and clinical support team.
- •
Continuous pipeline of next-generation, technologically superior products.
- •
Deep, collaborative relationships with key opinion leaders (KOLs) in cardiology.
Double down on the current successful model. Re-invest capital from the Critical Care sale into R&D and pivotal clinical trials for TMTT and heart failure. Expand the specialized sales and clinical teams ahead of new product launches.
Prioritized Initiatives
- Initiative:
Accelerate TMTT Commercialization
Expected Impact:High
Implementation Effort:High
Timeframe:1-3 Years
First Steps:Secure US FDA approval for the EVOQUE valve. Finalize reimbursement coding and pricing. Scale up the dedicated TMTT commercial and clinical support teams in the US and EU.
- Initiative:
Drive TAVR Indication Expansion for Asymptomatic & Moderate AS
Expected Impact:High
Implementation Effort:Medium
Timeframe:1-2 Years
First Steps:Complete enrollment in the EARLY TAVR pivotal trial. Begin pre-launch market development activities with KOLs to educate the market on the new data and patient population.
- Initiative:
Systematic Market Penetration in China
Expected Impact:High
Implementation Effort:High
Timeframe:2-5 Years
First Steps:Secure NMPA approval for the latest SAPIEN and TMTT devices. Establish a local clinician training and education infrastructure. Build a dedicated direct sales organization.
Experimentation Plan
High Leverage Tests
{'test': "Pilot a 'Heart Valve Navigator' program at partner hospitals to test the impact on patient identification and time-to-treatment.", 'hypothesis': 'A dedicated navigator role will increase the conversion of diagnosed patients to treated patients by 20%.'}
{'test': 'Launch a targeted digital marketing campaign to caregivers of the elderly and measure the lift in traffic to aortic stenosis screening resources.', 'hypothesis': 'A caregiver-focused campaign will generate a higher click-through and screening completion rate than a patient-focused one.'}
Use a combination of quantitative metrics (procedure volumes, referral rates, marketing analytics) and qualitative feedback (physician and patient surveys) to measure the success of initiatives.
Quarterly review of ongoing growth experiments, with an annual strategic planning cycle to prioritize the next wave of initiatives.
Growth Team
A 'Strategic Market Development' model, embedded within each business unit (TAVR, TMTT). This team would be a cross-functional group of marketing, clinical affairs, health economics, and sales strategy professionals.
Key Roles
- •
Director of Market Access & Reimbursement (TMTT)
- •
Therapy Development Manager (New Geographies)
- •
Digital Patient Engagement Lead
- •
Health Economics & Outcomes Research (HEOR) Analyst
Invest in training the existing commercial teams on health economics and selling to hospital administrators. Recruit top talent from the digital health and data science sectors to build new capabilities internally.
Edwards Lifesciences is in an exceptionally strong position to accelerate growth. The recent strategic divestiture of its Critical Care business is a pivotal move, sharpening its focus and providing a $4.2 billion war chest to dominate the structural heart market.
The company's growth foundation is solid, anchored by a powerful product-market fit in TAVR, where it holds a commanding market share. It is operating in a market with powerful tailwinds, including an aging population and a clinical shift toward the minimally invasive procedures it pioneered.
The primary growth engine will shift from optimizing the maturing TAVR market to igniting the nascent, but potentially larger, Transcatheter Mitral and Tricuspid Therapies (TMTT) market. Success here is the single most critical factor for long-term growth and represents a massive opportunity. Key scale barriers are not capital-related but are operational and clinical in nature: the ability to scale physician training, navigate global reimbursement, and flawlessly execute complex clinical trials.
Growth opportunities are abundant and clear. The highest priorities must be: 1) commercializing the TMTT portfolio, 2) expanding TAVR into new patient populations, and 3) systematic geographic expansion into the Asia-Pacific region.
The recommended strategy is to double down on its proven 'Medical Innovation & Clinical Adoption' model. The North Star Metric should be 'Number of Patients Treated Annually,' which perfectly aligns its commercial goals with its patient-first mission. By redeploying capital and focus from the divestiture into its core competencies of R&D, clinical evidence generation, and physician education, Edwards Lifesciences is poised to not only defend its leadership in TAVR but also create and lead the next frontier of structural heart care.
Legal Compliance
Edwards Lifesciences maintains a comprehensive and easily accessible 'Privacy Statement'. It clearly articulates the types of personal and sensitive personal information collected, including health-related data submitted by patients or healthcare professionals. The policy details the purposes for data use, such as responding to inquiries, providing product information, and meeting regulatory reporting obligations like adverse event reporting to the FDA. It addresses international data transfers, acknowledging its global operations and reliance on mechanisms like Standard Contractual Clauses for data transfers from the EEA, UK, and Switzerland. The policy also mentions that they do not sell personal information for third-party marketing purposes. Specific sections address data subject rights and provide contact information for privacy-related inquiries, demonstrating a robust framework. However, the policy could be enhanced by providing more region-specific details (e.g., a dedicated GDPR or CCPA/CPRA section with explicit rights) rather than integrating them into a single global document.
The 'Legal Terms' document is present and clearly accessible. It contains several crucial disclaimers appropriate for the medical technology industry. A key strength is the prominent disclaimer stating that website content is for informational purposes only and is not a substitute for professional medical advice. This is a critical risk mitigation strategy. The terms also include standard clauses on liability limitations ('AS IS' basis), intellectual property rights, and rules for user conduct. It clearly states that Edwards is not responsible for third-party site content linked from their website. While comprehensive, the document is dense and could benefit from clearer headings and a summary section to improve readability for the average user.
Upon visiting the website, a cookie consent banner is immediately displayed. The banner provides clear options to 'Accept All Cookies', 'Reject All', or manage 'Cookie Settings'. This granular control is a best practice under GDPR and other modern privacy laws. Allowing users to reject non-essential cookies before they are placed is a key compliance strength. The mechanism appears to effectively block tracking technologies prior to user consent, which is a significant positive from a legal positioning standpoint.
Edwards Lifesciences demonstrates a strong commitment to data protection, reflective of its position in the highly regulated healthcare sector. The privacy policy covers key requirements under both GDPR and CCPA/CPRA, such as the legal basis for processing, data retention, security measures, and data subject rights (access, deletion, etc.). The company explicitly states it will not discriminate against users for exercising their privacy rights. They acknowledge the collection of sensitive health information and specify that consent will be obtained where required. The global nature of their data processing is transparently disclosed, along with the legal safeguards used for international transfers. A dedicated 'Declaration for California Compliance Law' further strengthens their position in a key US market.
The website does not appear to have a dedicated 'Accessibility Statement' in the primary footer, which is a missed opportunity to signal commitment to inclusivity. A full technical audit is beyond this scope, but a preliminary review shows good practices like clear navigation structures and legible font choices. However, for a healthcare-related company, where users may have diverse abilities, proactive adherence and public commitment to WCAG (Web Content Accessibility Guidelines) standards are crucial. In the US, healthcare entities receiving federal funds are often required to meet WCAG standards, making this a potential legal risk. Failing to ensure the website is accessible to people with disabilities could create exposure to litigation under the Americans with Disabilities Act (ADA).
This is a key strength in Edwards' legal positioning. The website effectively segregates content for 'Patients' and 'Healthcare Professionals'. This is critical for complying with FDA and EU MDR regulations regarding the promotion and advertising of medical devices. Such regulations often have different rules for direct-to-consumer information versus materials intended for medical experts. All promotional claims for medical devices must be consistent with their FDA-cleared or CE-marked intended use, and the website's content appears carefully managed to avoid off-label promotion. The 'Legal Terms' appropriately disclaim that the site does not offer medical advice, which is essential for managing liability. They also transparently disclose requirements to report certain information (e.g., adverse events) to regulatory bodies like the FDA, integrating compliance directly into their data privacy framework.
Compliance Gaps
- •
Absence of a dedicated and easily findable 'Accessibility Statement' detailing commitment and conformance to WCAG 2.1 AA standards.
- •
Privacy Policy, while comprehensive, lacks distinct, easy-to-navigate sections for major regulatory regimes like GDPR and CCPA/CPRA, which can make it difficult for users in those regions to understand their specific rights.
- •
The 'Legal Terms' are lengthy and legally dense, which could be challenging for patient audiences to understand, potentially impacting the enforceability of certain clauses.
- •
The website does not explicitly state its position on HIPAA compliance. While as a device manufacturer it may not always be a 'Covered Entity', interactions with healthcare providers could make it a 'Business Associate', creating HIPAA obligations for any Protected Health Information (PHI) handled.
Compliance Strengths
- •
Strong, granular cookie consent mechanism with clear 'Accept All', 'Reject All', and 'Settings' options.
- •
Excellent audience segmentation between 'Patients' and 'Healthcare Professionals', which is a cornerstone of medical device marketing compliance.
- •
Comprehensive and transparent Privacy Statement that covers global data transfers and the handling of sensitive health data.
- •
Prominent and clear disclaimer in the 'Legal Terms' that website information is not a substitute for professional medical advice, a critical risk-management tool.
- •
Specific declaration of compliance with California's transparency law for the pharmaceutical and medical device industry, showing proactive state-level compliance.
- •
Provides a confidential and anonymous 'Integrity Helpline' for reporting compliance concerns.
Risk Assessment
- Risk Area:
Website Accessibility (ADA/WCAG)
Severity:High
Recommendation:Commission a third-party WCAG 2.1 AA audit. Based on the findings, remediate all identified accessibility issues. Publish a formal 'Accessibility Statement' on the website, outlining the company's commitment and providing a channel for users to report issues.
- Risk Area:
HIPAA Compliance Ambiguity
Severity:Medium
Recommendation:Clarify within the Privacy Policy or a separate notice the company's status and obligations under HIPAA, particularly in scenarios where it may act as a 'Business Associate' to healthcare providers. This manages expectations and reduces potential liability.
- Risk Area:
Privacy Policy Readability
Severity:Low
Recommendation:Restructure the Privacy Statement with region-specific sections (e.g., 'Information for European Users', 'Your California Privacy Rights') and use layered notices or expandable sections to improve navigation and comprehension for non-expert audiences.
- Risk Area:
Terms of Service Clarity
Severity:Low
Recommendation:Add a 'plain language' summary at the beginning of the 'Legal Terms' to highlight key points for users, especially patients, regarding disclaimers, liability, and proper use of website information.
High Priority Recommendations
- •
Immediately address website accessibility by commissioning a WCAG 2.1 AA audit and creating a public-facing Accessibility Statement to mitigate significant legal risk under the ADA.
- •
Enhance the Privacy Statement by adding distinct, user-friendly sections for GDPR and CCPA/CPRA to provide greater clarity and demonstrate direct compliance with major data protection laws.
- •
Conduct an internal review to define and disclose the company's position and compliance posture regarding HIPAA, especially in contexts where it could be considered a Business Associate.
Edwards Lifesciences has established a sophisticated and robust legal compliance framework for its corporate website, reflecting its status as a global leader in the highly regulated medical technology industry. The company's strategic positioning is particularly strong in the areas of medical device marketing regulations and data privacy. The clear demarcation of content for patients and healthcare professionals is a best-in-class approach to mitigating risks associated with off-label promotion under FDA and EU MDR rules. Furthermore, the cookie consent mechanism and the detailed Privacy Statement demonstrate a serious commitment to global data protection standards, which builds customer and partner trust.
However, the most significant legal risk lies in website accessibility. The lack of a clear, public commitment to WCAG standards presents a material risk of litigation under the ADA and may alienate a segment of its patient user base. This gap is inconsistent with the company's patient-focused mission. While the existing privacy and terms documents are legally comprehensive, their usability could be improved for a diverse global audience that includes patients who may not have legal expertise. Addressing the high-priority recommendations—particularly accessibility—will not only close a critical compliance gap but also enhance the company's brand reputation as a truly patient-centric organization.
Visual
Design System
Professional & Empathetic Corporate
Excellent
Advanced
User Experience
Navigation
Horizontal Mega Menu
Intuitive
Excellent
Information Architecture
Logical
Clear
Light
Conversion Elements
- Element:
Text Link CTA ('More about us ->')
Prominence:Low
Effectiveness:Somewhat effective
Improvement:Convert text links into styled, high-contrast buttons (e.g., using the brand's red color) to increase visual weight and click-through rates. Add a subtle hover animation for better feedback.
- Element:
Audience-specific Links ('Patients and Care Partners', 'Healthcare Professionals')
Prominence:Medium
Effectiveness:Effective
Improvement:While effective, consider adding a brief, one-line descriptor under each link on hover to clarify the value proposition for each audience segment, further aiding navigation.
- Element:
External Site Link ('Visit treatheartvalvefailure.com ->')
Prominence:Low
Effectiveness:Ineffective
Improvement:This CTA is visually identical to internal links but directs users off-site. It should be clearly differentiated with an 'external link' icon and presented with more visual weight to manage user expectations and improve trust.
- Element:
Career-focused CTA ('Come to be inspired ->')
Prominence:Low
Effectiveness:Somewhat effective
Improvement:Recruitment is a key business goal. This CTA should be more prominent. A dedicated section with a background image and a clear button would be more effective than a simple text link at the bottom of the page.
Assessment
Strengths
- Aspect:
Patient-Centric Visual Storytelling
Impact:High
Description:The website effectively uses high-quality, emotive photography of a diverse range of patients. This approach builds an immediate emotional connection and powerfully communicates the company's mission to help people, fostering trust and brand loyalty.
- Aspect:
Clear Audience Segmentation
Impact:High
Description:The primary navigation clearly separates 'Patients & Care Partners' from 'Healthcare Professionals'. This is a critical strength for a MedTech company, as it allows for tailored user journeys and prevents users from being overwhelmed by irrelevant information.
- Aspect:
Clean & Professional Aesthetic
Impact:Medium
Description:The use of ample whitespace, a consistent color palette (red, grays, white), and clean typography creates a professional, credible, and trustworthy visual identity. This is essential for a company in the medical and healthcare sector.
- Aspect:
Structured Information Architecture
Impact:Medium
Description:The homepage is well-organized into logical, thematic blocks (e.g., 'Innovative solutions', 'Clinical research', 'Corporate Impact'). This scannable layout allows users to quickly understand the scope of the company and find relevant sections.
Weaknesses
- Aspect:
Low-Prominence CTAs
Impact:High
Description:The most critical calls-to-action are styled as simple text links with an arrow. They lack the visual prominence of buttons, which can lead to lower engagement and conversion on key user journeys like learning about solutions or visiting informational micro-sites.
- Aspect:
Understated Product Showcase
Impact:Medium
Description:The 'patient-focused innovative technologies' section shows small, static images of complex medical devices. This fails to convey the sophistication and life-saving nature of their products. Interactive elements, videos, or more detailed visuals could significantly enhance this section.
- Aspect:
Lack of Social Proof & Testimonials
Impact:Medium
Description:While patient photos are used, the homepage lacks direct testimonials, physician endorsements, or key statistics (e.g., 'over 1 million patients helped'). Adding this social proof would substantially boost credibility and trust for both patient and professional audiences.
- Aspect:
Vague 'About Us' CTA
Impact:Low
Description:The link text 'More about us' is generic. A more value-oriented CTA like 'Discover Our Mission' or 'See Our Life-Saving Work' would be more compelling and better aligned with the brand's impactful story.
Priority Recommendations
- Recommendation:
Redesign all primary CTAs from text links to high-contrast buttons.
Effort Level:Low
Impact Potential:High
Rationale:This is a fundamental conversion optimization principle. Button-style CTAs are significantly more noticeable and clickable than text links. Implementing this change will directly improve user flow into key product, patient, and corporate information sections, boosting engagement.
- Recommendation:
Integrate a 'Patient Stories' or 'Clinician Impact' section on the homepage.
Effort Level:Medium
Impact Potential:High
Rationale:Adding direct quotes or short video testimonials will add a powerful layer of social proof and authenticity. This strengthens the emotional connection established by the photography and provides tangible evidence of the company's positive impact, building trust with all audience segments.
- Recommendation:
Enhance the product showcase with interactive elements.
Effort Level:High
Impact Potential:Medium
Rationale:For healthcare professionals and curious patients, understanding the technology is key. Replacing static images with 3D models, short animated videos explaining the mechanism, or interactive hotspots on the devices would better communicate the innovation and sophistication of Edwards' products.
- Recommendation:
Differentiate external vs. internal link CTAs visually.
Effort Level:Low
Impact Potential:Medium
Rationale:Clearly indicating when a link will take a user to a different website (e.g., treatheartvalvefailure.com) is a UX best practice that builds trust and prevents user confusion. A simple 'external link' icon next to the CTA is sufficient to manage expectations effectively.
Mobile Responsiveness
Excellent
The design adapts seamlessly across major breakpoints. On mobile, the navigation collapses into a clear hamburger menu, content blocks stack logically in a single column, and font sizes are appropriately scaled for readability.
Mobile Specific Issues
No itemsDesktop Specific Issues
No itemsThis visual design audit of Edwards Lifsciences, a global leader in medical technology for structural heart disease, reveals a mature, professional, and highly effective digital presence that successfully balances corporate credibility with a deep sense of patient empathy.
Design System and Brand Identity:
The website employs an advanced and consistent design system. The brand's primary color, a strong red, is used sparingly but effectively for emphasis, such as in the hero banner's text overlay, creating an immediate focal point. The overall aesthetic is clean, modern, and corporate, instilling a sense of trust and scientific authority. The use of high-quality, emotionally resonant photography of patients is the cornerstone of their visual storytelling, perfectly aligning with their brand credo: "Helping patients is our life's work".
Visual Hierarchy and User Experience:
The information architecture is logical and serves its diverse audiences—primarily patients and healthcare professionals—exceptionally well through clear top-level navigation. The visual hierarchy on the homepage is effective, guiding the user through large, benefit-driven headlines from the company's mission to its specific areas of impact, such as clinical research and corporate giving. The use of generous whitespace and a clear typographic scale creates a light cognitive load, making the content easily scannable and digestible.
Conversion Elements and Actionable Recommendations:
The most significant area for improvement lies in the effectiveness of the calls-to-action (CTAs). Currently, primary CTAs are presented as understated text links (e.g., 'More about us ->'). While clean, this approach sacrifices visual prominence and likely suppresses click-through rates. The highest priority recommendation is to transform these text links into distinct, high-contrast buttons. This simple change will create clearer visual cues for users, guiding them more effectively toward key informational pathways and conversion goals.
Furthermore, the homepage would be greatly enhanced by the inclusion of direct social proof. While the patient photography is powerful, incorporating brief, impactful testimonials from patients or endorsements from clinicians would add a crucial layer of authenticity and trust. A section dedicated to 'Patient Stories' or 'Clinician Impact' could powerfully substantiate the claims made throughout the site. Finally, the product showcase is currently too static; it fails to capture the groundbreaking nature of the medical devices. Introducing interactive elements like 3D viewers or short video explainers would better communicate the company's technological prowess to its professional audience.
Discoverability
Market Visibility Assessment
Edwards Lifesciences holds a dominant brand authority position among healthcare professionals (HCPs), built on a long history of innovation and extensive clinical data. Digitally, this authority is manifested through a professional, data-centric website. However, its thought leadership is less visible to the broader public and patient audiences, creating an opportunity to translate its clinical leadership into more accessible educational content.
Edwards is a recognized leader in the Transcatheter Aortic Valve Replacement (TAVR) market, frequently cited alongside key competitors Medtronic and Abbott. Its digital visibility for core product terms like 'SAPIEN valve' is strong among clinicians. However, for broader disease-state terms like 'aortic stenosis treatment', it competes for visibility with major medical institutions (e.g., Mayo Clinic) and its direct competitors. Recent clinical trial data shows a competitive landscape where Edwards' SAPIEN and Medtronic's Evolut are primary contenders.
The digital potential for customer acquisition is high but distinctly segmented. For HCPs, the website serves as a crucial resource for clinical trial data, product specifications, and training information, directly influencing adoption and purchasing decisions by hospitals and surgical teams. For patients, the potential lies in driving disease awareness and encouraging proactive conversations with doctors, as seen with their dedicated patient site. This patient-driven demand can significantly influence which devices are considered.
As a global company operating in over 100 countries, Edwards has a significant physical market presence. Its primary digital property, edwards.com, appears to be U.S.-centric, though it serves a global audience. A key strategic opportunity is to enhance digital penetration through localized content, catering to regional regulatory environments, clinical practices, and language preferences to better support international sales and adoption.
The website provides comprehensive coverage of its core business areas: transcatheter and surgical therapies for aortic, mitral, and tricuspid valve diseases. The content is robust for its flagship products. However, there is an opportunity to expand topic coverage to earlier stages of cardiovascular disease, focusing on diagnosis and disease progression, which could capture potential patients and referring physicians earlier in their journey.
Strategic Content Positioning
Edwards effectively segments its content for its two primary audiences: 'Healthcare Professionals' and 'Patients.' The HCP content aligns well with the consideration and decision stages, providing deep clinical evidence. The patient content, including the separate treatheartvalvefailure.com site, effectively targets the awareness and education stages. The strategy could be enhanced by creating more content that bridges the gap between patient education and the clinical decision-making process, empowering patients for discussions with their doctors.
While Edwards is a clinical leader, its digital thought leadership can be amplified. Opportunities include creating a centralized, public-facing hub for all clinical research, developing accredited webinar series for HCPs featuring key opinion leaders, and producing high-quality, accessible video content that explains complex cardiovascular conditions to patients and caregivers. This would solidify their position as the definitive educational resource in structural heart disease.
Competitors like Medtronic and Abbott also invest heavily in HCP and patient education. A key gap for Edwards to exploit is the area of digital support and training tools for surgical teams. Developing an exclusive online portal with surgical simulations, advanced case studies, and peer-to-peer collaboration tools could create a significant competitive moat. Additionally, focusing on the long-term patient experience and quality of life post-procedure with dedicated content could build a stronger patient-brand connection than competitors.
The core brand message of being 'driven by a passion to help patients' is consistently communicated across the corporate website, from the homepage to the 'About Us' and 'Corporate Impact' sections. This patient-first messaging is a strong asset that resonates with both clinicians and patients, reinforcing the company's mission-driven culture.
Digital Market Strategy
Market Expansion Opportunities
- •
Develop comprehensive educational platforms for adjacent or earlier-stage cardiovascular conditions to broaden brand authority and capture new patient populations.
- •
Create region-specific content hubs for key international markets (e.g., Europe, Japan) to address local clinical needs and regulatory landscapes, supporting global growth.
- •
Target allied health professionals, such as cardiac nurses and physician assistants, with tailored educational content to build a wider network of brand advocates within hospital systems.
Customer Acquisition Optimization
- •
Implement sophisticated content funnels for HCPs based on specialty (e.g., interventional cardiologist vs. cardiac surgeon) to deliver highly relevant clinical data and shorten the adoption cycle.
- •
Expand patient-focused, unbranded disease awareness campaigns to grow the overall market of treated patients, thereby increasing demand for Edwards' solutions.
- •
Create a seamless digital experience for HCPs to access training materials, request support, and order products, reducing friction and improving loyalty.
Brand Authority Initiatives
- •
Launch a definitive online 'Clinical Evidence Library' consolidating all publications, trial data, and expert analyses, making it the go-to resource for structural heart research.
- •
Produce a high-quality video or podcast series featuring leading cardiologists discussing innovations and patient outcomes, positioning Edwards as a convener of experts.
- •
Systematically gather and showcase (with permission) patient testimonials focusing on long-term quality of life, building trust and emotional connection with future patients.
Competitive Positioning Improvements
- •
Develop content that clearly articulates the unique long-term clinical and economic benefits of their proprietary technologies, such as the RESILIA tissue, to differentiate from competitors.
- •
Position Edwards not merely as a device manufacturer but as a strategic partner to hospitals in establishing and optimizing their structural heart programs through digital tools and resources.
- •
Leverage superior clinical outcomes from head-to-head trials (where applicable) in HCP-facing marketing to directly address competitive threats from Medtronic, Abbott, and others.
Business Impact Assessment
Digital market share can be tracked via proxy metrics such as share-of-voice in organic search for key non-branded terms (e.g., 'TAVR', 'mitral valve repair'), referral traffic from top-tier medical journals and societies, and engagement rates on HCP-focused platforms like LinkedIn.
For HCPs, success is measured by downloads of clinical papers, webinar registrations, and leads generated for sales reps. For patients, metrics include traffic to educational portals, video engagement rates on patient stories, and use of 'Find a Specialist' tools. The overarching goal is to increase the velocity of qualified leads for both audiences.
Authority is measured by branded search volume growth, citations of Edwards' research in external publications, media mentions in leading medical news outlets, and the quantity and quality of engagement from verified HCPs on social and professional networks.
Benchmarking involves regular analysis of competitor website traffic, content strategy, and social media engagement. Success is defined by achieving a superior share-of-voice for strategic keywords and demonstrating higher engagement rates on educational content compared to key competitors like Medtronic and Abbott.
Strategic Recommendations
High Impact Initiatives
- Initiative:
Develop a 'Clinical Partner Program' Digital Hub
Business Impact:High
Market Opportunity:Solidifies Edwards' role as an indispensable partner to hospitals, moving beyond a vendor relationship to improve retention and deepen integration into clinical workflows.
Success Metrics
- •
Number of hospital systems enrolled
- •
Usage of digital training modules and support tools
- •
Reduction in sales cycle length
- Initiative:
Launch a Global Patient & Caregiver Education Platform
Business Impact:High
Market Opportunity:Builds a powerful, direct-to-patient brand preference that influences treatment decisions and grows the overall market for structural heart therapies.
Success Metrics
- •
Monthly active users on the platform
- •
Growth in patient-initiated inquiries to physicians
- •
Branded search lift for patient-related terms
- Initiative:
Create a 'Future of Structural Heart' Thought Leadership Series
Business Impact:Medium
Market Opportunity:Positions Edwards at the forefront of innovation, attracting top clinical talent for trials and partnerships while reinforcing brand leadership.
Success Metrics
- •
Views and engagement from target HCPs
- •
Media mentions and citations
- •
Inbound inquiries for research collaboration
Transition from a 'product-centric' to a holistic 'clinical outcome and patient advocacy' digital strategy. This involves positioning Edwards not just as the manufacturer of the best devices, but as the essential partner for clinicians in advancing techniques and for patients in navigating their entire treatment journey. This elevates the brand beyond product features and builds a defensible, long-term market advantage.
Competitive Advantage Opportunities
- •
Leverage the extensive library of long-term clinical data to create the most comprehensive and trusted evidence-based resource online, making it a primary destination for clinical research.
- •
Build best-in-class digital training and support platforms for clinicians, making the adoption and continued use of Edwards' products easier and more effective than competitors'.
- •
Foster a direct, trusted relationship with the patient community through unparalleled educational resources and support, creating a powerful brand preference that physicians cannot ignore.
Edwards Lifesciences is a recognized clinical leader in the structural heart market, and its digital presence reflects this with a strong, professional foundation targeted at healthcare professionals. The company's primary strength lies in its deep well of clinical evidence, which underpins its authority and credibility. The current digital strategy effectively bifurcates the audience, providing distinct pathways for clinicians and patients. However, a significant strategic opportunity exists to evolve beyond this segmented approach into a more integrated ecosystem. The company's key competitors, Medtronic and Abbott, are also formidable players with sophisticated marketing efforts, making digital differentiation crucial.
The primary strategic recommendation is to shift the digital market position from that of a premier device manufacturer to that of an indispensable clinical partner and patient advocate. For clinicians, this means creating digital tools, training platforms, and data resources so valuable that they become integral to their practice. For patients, it means building an educational and support community that empowers them from diagnosis through recovery, fostering a level of brand trust and preference that directly influences treatment decisions. By focusing on the entire care continuum and serving the needs of the entire ecosystem, Edwards can build a competitive moat that is not based solely on product innovation but on the deep, trusted relationships forged through its digital presence.
Strategic Priorities
Strategic Priorities
- Title:
Accelerate TMTT Commercialization to Establish a Second Growth Pillar
Business Rationale:The business is overly reliant on the maturing TAVR market. The Transcatheter Mitral and Tricuspid Therapies (TMTT) market represents a multi-billion dollar, underserved opportunity. Aggressively capturing leadership in this nascent market is critical for long-term revenue diversification and growth.
Strategic Impact:Transforms Edwards from a TAVR-dominant company into a diversified structural heart leader. Creates a second major revenue engine, reducing market concentration risk and unlocking the next phase of significant enterprise growth.
Success Metrics
- •
TMTT revenue as a percentage of total sales (target 20-25% within 5 years)
- •
Market share leadership in key TMTT segments (e.g., transcatheter mitral replacement)
- •
Number of hospital systems adopting the full TMTT portfolio
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Revenue Model
- Title:
Launch a 'Clinical Partner' Digital Ecosystem
Business Rationale:Competitors offer devices, but the opportunity lies in becoming an indispensable partner to hospital 'Heart Teams'. Developing a digital platform integrating AI-powered procedural planning, training modules, and outcomes tracking moves the relationship beyond a transactional one.
Strategic Impact:Creates a significant competitive moat based on workflow integration and data, not just device features. Increases customer stickiness, improves patient outcomes, and provides a new, high-margin software-as-a-service or value-add revenue stream.
Success Metrics
- •
Adoption rate of the digital platform by top-tier hospital partners
- •
Reduction in physician training time and costs
- •
Correlation of platform usage with improved clinical outcomes and efficiency
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Customer Strategy
- Title:
Execute Pivotal Trials to Expand TAVR into New Patient Populations
Business Rationale:To maintain growth in the core TAVR business, the total addressable market must expand. The most significant opportunity is gaining indications for lower-risk, younger, and asymptomatic patient populations, which requires flawless execution of landmark clinical trials.
Strategic Impact:Fundamentally shifts clinical guidelines and solidifies TAVR as the standard of care for a much broader patient base. Unlocks the next major wave of growth for the company's primary revenue source, defending against competitive commoditization.
Success Metrics
- •
Positive pivotal trial outcomes (e.g., EARLY TAVR)
- •
Inclusion in updated ACC/AHA and ESC clinical guidelines
- •
Year-over-year growth in procedure volumes in newly indicated populations
Priority Level:HIGH
Timeline:Long-term Vision (12+ months)
Category:Market Position
- Title:
Reposition the Brand from 'Passionate Innovator' to 'Proven Global Leader'
Business Rationale:The current brand messaging focuses on passion but fails to assert the company's documented market leadership and unmatched clinical evidence. This creates a perception gap that competitors can exploit. The brand narrative must be fortified with proof to substantiate its premium position.
Strategic Impact:Aligns market perception with reality, strengthening pricing power and reinforcing clinician trust. Creates a more formidable brand that proactively defends market share and clearly communicates why Edwards is the 'gold standard' partner for structural heart programs.
Success Metrics
- •
Increased share-of-voice for leadership-focused messaging vs. competitors
- •
Measurable lift in brand perception surveys among clinicians regarding 'clinical evidence' and 'market leadership'
- •
Reduction in pricing pressure during hospital contract negotiations
Priority Level:MEDIUM
Timeline:Quick Win (0-3 months)
Category:Brand Strategy
- Title:
Systematize Market Penetration in the Asia-Pacific (APAC) Region
Business Rationale:While strong in the US and Europe, the business has significant room for growth in the APAC region, which has a rapidly aging population and rising healthcare investment. A dedicated, systematic strategy is required to navigate local regulatory bodies, establish training infrastructure, and build commercial channels.
Strategic Impact:Unlocks a major new geographic revenue stream, balancing the portfolio against mature Western markets. Establishes a long-term growth vector that can fuel the business for the next decade.
Success Metrics
- •
Year-over-year revenue growth in key APAC markets (China, Japan)
- •
Time-to-approval for new devices from local regulatory bodies (e.g., NMPA in China)
- •
Number of trained, actively-implanting physicians in the region
Priority Level:MEDIUM
Timeline:Long-term Vision (12+ months)
Category:Operations
Edwards Lifesciences must transition from being a TAVR-focused device manufacturer to the undisputed, full-portfolio leader in structural heart solutions. This requires defending its TAVR dominance by expanding into new patient populations while aggressively commercializing its TMTT pipeline to establish a second, powerful growth engine.
The core competitive advantage to build upon is the unparalleled depth of long-term clinical evidence. This body of proof is the most defensible asset, justifying premium pricing, building profound clinician trust, and creating a formidable barrier to competitor entry.
The primary growth catalyst is the successful commercialization of the Transcatheter Mitral and Tricuspid Therapies (TMTT) portfolio. Capturing leadership in this nascent, multi-billion-dollar market will fuel the company's next decade of growth and solidify its overall market dominance.