eScore
gilead.comThe eScore is a comprehensive evaluation of a business's online presence and effectiveness. It analyzes multiple factors including digital presence, brand communication, conversion optimization, and competitive advantage.
Gilead's digital presence is highly authoritative, with a strong domain rating and excellent content alignment for professional audiences like HCPs and investors. The site is well-structured and clearly serves its primary purpose as a corporate and scientific information hub. However, its multi-channel presence is less focused on broad public engagement, and content could be better optimized for early-stage, non-branded search queries to capture a wider audience.
Exceptional content authority and search intent alignment for its core scientific and investor audiences.
Expand top-of-funnel content strategy to build authority around unbranded disease-state topics in growth areas like oncology and inflammation, capturing a broader audience.
The brand messaging is exceptionally clear, professional, and consistent, effectively positioning Gilead as a science-first innovator. It successfully tailors messages to investors and scientists by highlighting pipeline data and innovation. However, the communication is overly corporate and lacks a strong emotional connection, missing the opportunity to feature patient voices which would better demonstrate the human impact of its therapies.
A clear, consistent, and authoritative brand voice that effectively communicates its scientific leadership and innovation focus to professional audiences.
Humanize the brand by integrating patient-centric narratives and stories on the homepage to bridge the emotional gap between corporate science and real-world human impact.
As a non-commercial site, 'conversion' is about guiding specific audiences to key information. The site's clear information architecture and navigation are effective at this for professionals. However, the analysis reveals inconsistent CTA design, which creates friction, and a significant weakness in digital accessibility, which presents both a compliance risk and a barrier for a portion of its user base.
A logical and well-structured information architecture that allows diverse professional audiences to efficiently navigate to relevant content areas like 'Pipeline' and 'Investors'.
Prioritize a formal digital accessibility audit against WCAG 2.1 AA standards and publish a global accessibility statement to mitigate legal risk and improve user experience for all.
Gilead demonstrates a sophisticated and mature approach to credibility and risk management, essential for the biopharma industry. The website features robust legal disclaimers, granular privacy policies, and transparent investor information, showcasing strong SEC and PhRMA code adherence. Third-party validation is evident through prominent news sections and clinical data, building a very high level of trust with its target audiences.
Robust, best-in-class legal and regulatory compliance framework integrated into the website, effectively managing risks related to drug promotion and securities law.
Revise the Terms of Use clause regarding user-submitted information to better align with user privacy expectations, enhancing trust without significantly increasing legal risk.
Gilead's competitive moat is wide and sustainable, built upon market dominance in HIV with blockbuster drugs like Biktarvy and a deep R&D pipeline to manage lifecycle threats. Its strategic acquisition of Kite Pharma established a strong, defensible leadership position in the high-growth, high-barrier field of cell therapy. The primary challenge is leveraging these strengths to diversify away from its heavy revenue dependence on the HIV franchise.
Deeply entrenched market dominance in the HIV space, combined with a pioneering, leadership position in the complex, high-barrier field of CAR T-cell therapy.
Accelerate clinical and commercial progress in the oncology pipeline to build a second revenue pillar of a comparable scale to the HIV franchise, mitigating concentration risk.
The business model is highly scalable due to the high operating leverage of patented pharmaceuticals, where revenue grows significantly faster than production costs. Gilead shows strong expansion signals through a clear strategy of M&A to enter new therapeutic areas and acquire next-generation platforms like in-vivo cell therapy. This proactive approach to acquiring innovation ensures a pipeline of future growth opportunities.
A proven and aggressive 'Science-Led M&A' strategy that allows the company to efficiently acquire new technologies and late-stage assets to enter high-growth markets.
Address the operational bottleneck of manufacturing complexity for cell therapies by investing in process automation and fully operationalizing the newly acquired in-vivo platforms.
Gilead's business model is exceptionally coherent, using its massive, predictable cash flow from the HIV franchise to fund diversification into high-growth areas like oncology. This strategy of defending the core while aggressively building the future is well-articulated and consistently executed. Strategic acquisitions are well-aligned with the stated goals of becoming a leader in oncology and inflammation.
Excellent strategic focus, using the highly profitable and dominant HIV franchise as a powerful engine to fund a clear, disciplined diversification strategy into oncology and inflammation.
Continue to improve revenue diversification to reduce the business model's high dependency on the HIV franchise, which remains the single largest systemic risk.
Gilead exhibits formidable market power, anchored by its dominant share of the global HIV market, which provides significant pricing power and leverage with payers. The company has demonstrated its ability to shape the market by evolving treatment paradigms from complex regimens to single-tablet solutions. Its influence is further solidified by a deep R&D pipeline and a history of transformative acquisitions that preempt competitive threats.
Unparalleled market share and pricing power in the HIV therapeutic area, which provides immense financial resources and a strategic foundation to out-invest competitors.
Counter the aggressive competitive messaging from rivals in the HIV prevention space by more forcefully promoting the unique benefits and access programs for its next-generation long-acting therapies.
Business Overview
Business Classification
Biopharmaceutical
Research & Development (R&D) Driven
Pharmaceuticals & Biotechnology
Sub Verticals
- •
Virology (HIV, Viral Hepatitis, COVID-19)
- •
Oncology (including Cell Therapy)
- •
Inflammation
Mature
Maturity Indicators
- •
Dominant market share in a core therapeutic area (HIV)
- •
Portfolio of multiple blockbuster drugs generating substantial, consistent revenue
- •
Robust global commercialization infrastructure
- •
Active M&A strategy to acquire new technology and pipeline assets (e.g., Kite Pharma, Interius BioTherapeutics)
- •
Deep and diverse clinical pipeline with over 50 programs
Enterprise
Steady
Revenue Model
Primary Revenue Streams
- Stream Name:
Product Sales: HIV Franchise
Description:Sale of patented antiretroviral drugs for the treatment and prevention of HIV. This is the company's largest and most established revenue source, led by the blockbuster drug Biktarvy.
Estimated Importance:Primary
Customer Segment:Patients with HIV, Healthcare Providers, Payers
Estimated Margin:High
- Stream Name:
Product Sales: Oncology Franchise
Description:Sale of cancer treatments, including cell therapies (Yescarta, Tecartus) from its Kite Pharma subsidiary and other oncology drugs like Trodelvy. This is a key strategic growth area for revenue diversification.
Estimated Importance:Secondary
Customer Segment:Cancer Patients, Oncologists & Hospitals, Payers
Estimated Margin:High
- Stream Name:
Product Sales: Liver Disease & Other Virology
Description:Sale of drugs for viral hepatitis (HCV, HBV) and other viral diseases, including the COVID-19 treatment Veklury (remdesivir). While historically significant, HCV revenue has declined, and Veklury sales are dependent on pandemic trends.
Estimated Importance:Tertiary
Customer Segment:Patients with Liver/Viral Diseases, Healthcare Providers, Payers
Estimated Margin:High
- Stream Name:
Licensing and Royalties
Description:Revenue generated from licensing agreements and collaborations with other pharmaceutical companies for the development and commercialization of its compounds or technologies.
Estimated Importance:Tertiary
Customer Segment:Other Biopharmaceutical Companies
Estimated Margin:High
Recurring Revenue Components
Chronic disease treatments (e.g., HIV) requiring lifelong therapy
Pricing Strategy
Value-Based Pharmaceutical Pricing
Premium
Opaque
Pricing Psychology
Not applicable in the traditional B2C sense; pricing is driven by clinical value, R&D investment, and negotiations with institutional payers.
Monetization Assessment
Strengths
- •
Strong patent protection on key products enables high-profit margins.
- •
Dominant market position in the chronic HIV care market provides a highly predictable and substantial cash flow.
- •
Diversifying portfolio into high-growth oncology market.
Weaknesses
High dependency on the HIV franchise creates significant revenue concentration risk.
Upcoming patent expirations ('patent cliff') for major drugs pose a threat to future revenue streams.
Opportunities
- •
Launch of long-acting injectables for HIV prevention and treatment (e.g., Lenacapavir) to extend franchise lifecycle and capture new market segments.
- •
Continued expansion in oncology through R&D and strategic acquisitions, such as moving into in-vivo cell therapy.
- •
Leveraging the cell therapy platform (Kite) for autoimmune diseases.
Threats
- •
Increasing competition in both HIV (e.g., ViiV Healthcare) and Oncology (e.g., Bristol Myers Squibb, Novartis).
- •
Intensifying pricing pressure and reimbursement hurdles from governments and private payers.
- •
Risk of clinical trial failures for key pipeline candidates.
Market Positioning
Scientific leadership and innovation in core therapeutic areas, with a dominant position in virology and a rapidly growing presence as a leader in cell therapy.
Dominant in HIV (estimated over 60% of the branded market). Growing challenger in cell therapy and oncology.
Target Segments
- Segment Name:
Patients
Description:Individuals diagnosed with life-threatening or chronic diseases such as HIV, cancer, viral hepatitis, and inflammatory conditions who require advanced medical treatments.
Demographic Factors
Varies by disease state
Psychographic Factors
- •
Seeking effective treatments with improved quality of life
- •
Concerned with long-term health management
- •
Value convenience and reduced side effects
Behavioral Factors
Adherence to long-term medication regimens
Interaction with specialist healthcare providers
Pain Points
- •
Lack of effective or curative treatment options
- •
Burdensome treatment regimens (e.g., daily pills)
- •
Significant side effects from medication
- •
High cost of care and access issues
Fit Assessment:Excellent
Segment Potential:High
- Segment Name:
Healthcare Providers
Description:Specialist physicians (e.g., infectious disease specialists, oncologists), hospitals, and clinics that diagnose, treat, and manage patients.
Demographic Factors
Medical professionals with prescribing authority
Psychographic Factors
- •
Data-driven and evidence-based
- •
Value clinical efficacy and patient safety
- •
Seek to provide the best standard of care
Behavioral Factors
- •
Attend medical conferences
- •
Read clinical journals
- •
Influence treatment guidelines
Pain Points
- •
Need for superior treatment options to improve patient outcomes
- •
Managing complex diseases and treatment side effects
- •
Keeping up-to-date with rapid medical innovation
- •
Navigating reimbursement and payer approvals
Fit Assessment:Excellent
Segment Potential:High
- Segment Name:
Payers
Description:Public and private entities that finance healthcare, including government health systems (e.g., Medicare), insurance companies, and pharmacy benefit managers (PBMs).
Demographic Factors
Large institutional entities
Psychographic Factors
Focus on cost-effectiveness and budget impact
Value data on long-term outcomes and health economics
Behavioral Factors
Negotiate drug prices and rebates
Develop drug formularies and coverage policies
Pain Points
- •
Managing escalating costs of specialty drugs
- •
Evaluating the long-term value of innovative but high-cost therapies
- •
Balancing patient access with budget constraints
Fit Assessment:Good
Segment Potential:Medium
Market Differentiation
- Factor:
Dominant HIV Portfolio
Strength:Strong
Sustainability:Sustainable
- Factor:
Leadership in Cell Therapy
Strength:Strong
Sustainability:Sustainable
- Factor:
Robust R&D and Clinical Pipeline
Strength:Strong
Sustainability:Sustainable
- Factor:
Global Commercial Infrastructure
Strength:Strong
Sustainability:Sustainable
Value Proposition
To discover, develop, and deliver innovative and transformative medicines for people with life-threatening diseases, turning impossible into possible.
Excellent
Key Benefits
- Benefit:
Transforming fatal diseases (like HIV) into manageable chronic conditions
Importance:Critical
Differentiation:Unique
Proof Elements
Market-leading single-tablet HIV regimens (e.g., Biktarvy)
Long history of innovation in antiretroviral therapy
- Benefit:
Providing curative therapies for diseases like Hepatitis C
Importance:Critical
Differentiation:Somewhat unique
Proof Elements
Portfolio of HCV drugs like Sovaldi and Harvoni
- Benefit:
Offering novel, personalized cancer treatments through cell therapy
Importance:Critical
Differentiation:Unique
Proof Elements
Approved CAR T-cell therapies (Yescarta, Tecartus)
Acquisition of next-generation in-vivo platforms
Unique Selling Points
- Usp:
Unparalleled expertise and market leadership in HIV treatment and prevention, now evolving into long-acting formulations.
Sustainability:Long-term
Defensibility:Strong
- Usp:
Pioneering position in the complex field of cell therapy via its Kite subsidiary, with the world's largest cell therapy manufacturing network.
Sustainability:Long-term
Defensibility:Strong
Customer Problems Solved
- Problem:
HIV was a fatal diagnosis with a complex, high pill-burden treatment.
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Limited treatment options for patients with certain refractory blood cancers.
Severity:Critical
Solution Effectiveness:Partial
- Problem:
Risk of HIV infection for high-risk populations.
Severity:Major
Solution Effectiveness:Complete
Value Alignment Assessment
High
Gilead's focus on areas with significant unmet medical need (HIV, oncology, virology) aligns perfectly with global healthcare priorities and market demand.
High
The value proposition of life-saving or life-altering medicines directly addresses the most critical needs of patients and the healthcare providers who treat them.
Strategic Assessment
Business Model Canvas
Key Partners
- •
Academic and Research Institutions (e.g., University of Pennsylvania)
- •
Biotechnology Companies (for licensing and acquisitions, e.g., Compugen, Arcellx).
- •
Regulatory Agencies (FDA, EMA)
- •
Payers and Government Health Systems
- •
Healthcare Providers and Hospital Networks
- •
Pharmaceutical Distributors
Key Activities
- •
Research & Development (R&D)
- •
Clinical Trial Management
- •
Drug Manufacturing & Supply Chain
- •
Regulatory Affairs & Market Access
- •
Sales & Marketing to Healthcare Professionals
Key Resources
- •
Intellectual Property (Patents)
- •
World-class scientific talent
- •
Global manufacturing and distribution network
- •
Strong brand reputation and market access
- •
Significant cash flow for R&D and acquisitions
Cost Structure
- •
High R&D expenditures
- •
Costs of clinical trials
- •
Sales, General & Administrative (SG&A) expenses
- •
Cost of Goods Sold (COGS)
- •
Costs related to mergers and acquisitions
Swot Analysis
Strengths
- •
Dominant market leadership and brand recognition in HIV.
- •
Strong and consistent cash flow from blockbuster drugs like Biktarvy.
- •
Proven expertise in both internal R&D and strategic M&A to build pipeline (e.g., Pharmasset, Kite).
- •
Leadership position in the high-growth field of cell therapy.
Weaknesses
- •
Over-reliance on HIV product sales, creating revenue concentration risk.
- •
Geographic concentration with a majority of revenue from the U.S. market.
- •
Some pipeline assets in competitive areas like oncology face strong incumbents.
Opportunities
- •
Diversify revenue streams by expanding the oncology and inflammation portfolios.
- •
Extend the HIV franchise's longevity with next-generation long-acting injectables.
- •
Leverage the Kite cell therapy platform to expand into new indications, including autoimmune diseases.
- •
Strategic acquisition of innovative biotech companies to acquire new technologies like in-vivo cell therapy.
Threats
- •
Loss of exclusivity (patent cliffs) for key products leading to generic competition.
- •
Intensifying competition from major pharmaceutical rivals in all core therapeutic areas.
- •
Increasing global pricing pressure and scrutiny from payers and governments.
- •
Potential for clinical trial failures or unfavorable regulatory decisions.
Recommendations
Priority Improvements
- Area:
Revenue Diversification
Recommendation:Aggressively accelerate the oncology and inflammation pipelines through both continued R&D investment and strategic, bolt-on acquisitions to reduce dependency on the HIV franchise.
Expected Impact:High
- Area:
Lifecycle Management
Recommendation:Ensure successful commercial launch and market penetration of long-acting HIV prevention and treatment options (Lenacapavir) to protect and extend the core virology revenue base against future competition and patent expiries.
Expected Impact:High
- Area:
Operational Efficiency
Recommendation:Fully integrate recent acquisitions like Interius BioTherapeutics to create centers of excellence, maximizing synergies and accelerating the development of next-generation platforms like in-vivo cell therapy.
Expected Impact:Medium
Business Model Innovation
- •
Expand beyond therapeutics into complementary digital health solutions (e.g., patient adherence apps, remote monitoring) to create a more integrated care ecosystem.
- •
Develop more outcome-based and value-based pricing models with payers to secure market access and justify premium pricing for transformative therapies.
- •
Invest further in platform technologies (like Kite's in-vivo platform) that can be applied across multiple disease areas, creating a scalable and defensible innovation engine.
Revenue Diversification
- •
Continue to pursue strategic acquisitions of clinical-stage biotech companies with first-in-class or best-in-class assets in oncology and immunology.
- •
Expand partnerships and licensing deals to gain access to novel therapeutic modalities (e.g., mRNA, gene editing) without the full cost of internal development.
- •
Systematically explore expanding the application of existing platforms, such as cell therapy for autoimmune diseases, to open up entirely new, large markets.
Gilead Sciences operates a robust and highly profitable business model founded on scientific innovation, particularly its long-standing dominance in the virology market. This leadership, anchored by the blockbuster HIV drug Biktarvy, generates substantial and predictable cash flow, which the company strategically reinvests into a deep R&D pipeline and transformative acquisitions. The company is at a pivotal point in its strategic evolution. While its core HIV franchise is a formidable strength, it also represents a significant concentration risk, with looming patent cliffs and intensifying competition. Recognizing this, Gilead's strategy is focused on a critical twofold objective: defending and extending its HIV leadership through next-generation, long-acting therapies, while aggressively diversifying into high-growth areas, most notably oncology. The acquisition of Kite Pharma was a masterstroke, immediately positioning Gilead as a leader in the revolutionary field of cell therapy. The more recent move to acquire Interius BioTherapeutics signals a forward-looking strategy to lead the next wave of innovation with in-vivo therapies, which could solve the logistical and cost challenges of current cell therapies. Future success and sustained growth will be contingent on the clinical and commercial success of its pipeline candidates in oncology and inflammation, its ability to successfully launch and scale new HIV prevention platforms, and its skill in navigating an increasingly challenging global landscape of drug pricing and reimbursement.
Competitors
Competitive Landscape
Mature
Oligopoly
Barriers To Entry
- Barrier:
High R&D Costs and Long Development Timelines
Impact:High
- Barrier:
Intellectual Property (Patents)
Impact:High
- Barrier:
Stringent Regulatory Approval Processes (e.g., FDA, EMA)
Impact:High
- Barrier:
Economies of Scale in Manufacturing and Distribution
Impact:Medium
- Barrier:
Established Relationships with Healthcare Providers and Payers
Impact:Medium
Industry Trends
- Trend:
Shift towards Personalized Medicine and Biologics (e.g., Cell & Gene Therapy)
Impact On Business:Gilead is actively investing in this area, notably through its Kite Pharma subsidiary, to build future growth drivers beyond small-molecule drugs.
Timeline:Immediate
- Trend:
Focus on Long-Acting Formulations for Chronic Diseases
Impact On Business:Directly aligns with Gilead's strategy for its next-generation HIV portfolio (e.g., Lenacapavir) to counter future patent expirations.
Timeline:Immediate
- Trend:
Increasing Use of AI and Machine Learning in Drug Discovery
Impact On Business:Adoption is crucial to accelerate R&D timelines and reduce costs to remain competitive.
Timeline:Near-term
- Trend:
Growing Pricing Pressure and Scrutiny from Payers and Governments
Impact On Business:Impacts profitability and market access strategies, requiring robust value demonstration for new therapies.
Timeline:Immediate
- Trend:
Expansion into Emerging Markets
Impact On Business:Offers new revenue streams but requires navigating different regulatory and market access landscapes.
Timeline:Long-term
Direct Competitors
- →
GSK (ViiV Healthcare)
Market Share Estimate:Significant, but second to Gilead in the HIV market (~16% vs. Gilead's ~73% historically, though this is a key battleground).
Target Audience Overlap:High
Competitive Positioning:A primary challenger in the HIV space, focusing on 2-drug regimens and long-acting injectables to differentiate from Gilead's portfolio.
Strengths
- •
Strong portfolio of HIV treatments, including long-acting injectable options (Cabenuva, Apretude).
- •
Focused R&D in HIV through the specialized ViiV Healthcare unit.
- •
Aggressive strategy to challenge Gilead's market dominance with alternative treatment paradigms (2-drug vs. 3-drug regimens).
Weaknesses
- •
Currently holds a smaller market share in the HIV treatment space compared to Gilead.
- •
Faces a significant challenge in unseating Gilead's blockbuster, Biktarvy.
- •
Revenue from HIV is a smaller percentage of GSK's overall business compared to Gilead's dependency.
Differentiators
Pioneering long-acting injectable HIV treatments.
Emphasis on two-drug regimens as a potentially safer long-term option.
- →
AbbVie Inc.
Market Share Estimate:Major player in immunology and oncology; direct competitor in viral hepatitis (HCV) and growing competitor in oncology.
Target Audience Overlap:Medium
Competitive Positioning:A diversified biopharma leader with dominant franchises in immunology (Skyrizi, Rinvoq) and a strong, growing presence in oncology.
Strengths
- •
Blockbuster immunology portfolio provides massive cash flow for R&D and acquisitions.
- •
Strong and growing oncology pipeline (e.g., Imbruvica, Venclexta).
- •
Established global commercial infrastructure.
Weaknesses
Historically high reliance on Humira, now facing biosimilar competition.
Less dominant position in virology compared to Gilead.
Differentiators
Market leadership in immunology.
Broad portfolio spanning immunology, oncology, neuroscience, and aesthetics.
- →
Merck & Co., Inc.
Market Share Estimate:Oncology powerhouse with Keytruda, also competes in virology and vaccines.
Target Audience Overlap:Medium
Competitive Positioning:An R&D-driven leader in immuno-oncology, with a strong legacy in vaccines and a broad pharmaceutical portfolio.
Strengths
- •
Dominant position in immuno-oncology with the blockbuster drug Keytruda.
- •
Extensive global presence and a strong R&D pipeline.
- •
Diversified portfolio across oncology, vaccines, and other therapeutic areas.
Weaknesses
Increasing reliance on Keytruda for revenue growth.
Less established leadership in the specific HIV market compared to Gilead and GSK.
Differentiators
Unparalleled market leadership in immuno-oncology (PD-1 inhibitors).
Strong global vaccine business.
- →
Bristol Myers Squibb (BMS)
Market Share Estimate:Leader in oncology and immunology, direct competitor in cell therapy.
Target Audience Overlap:Medium
Competitive Positioning:A leader in innovative treatments for serious diseases, with strong franchises in oncology (Opdivo, Yervoy) and a significant presence in cell therapy (Abecma, Breyanzi).
Strengths
- •
Strong immuno-oncology portfolio.
- •
Key player in the CAR T-cell therapy space, directly competing with Gilead's Kite Pharma.
- •
Successful track record of strategic acquisitions (e.g., Celgene).
Weaknesses
Faces significant competition in its key markets, particularly oncology.
Patent expirations on key products are a persistent challenge.
Differentiators
Pioneering work in immuno-oncology.
Strong competitive portfolio in CAR T-cell therapies.
- →
Johnson & Johnson (Janssen)
Market Share Estimate:Major diversified player in pharmaceuticals, including oncology, immunology, and infectious diseases.
Target Audience Overlap:Medium
Competitive Positioning:A diversified healthcare giant with a strong pharmaceutical division focused on complex diseases across multiple therapeutic areas.
Strengths
- •
Extremely diversified business model reduces reliance on any single product.
- •
Strong pipeline and commercial presence in oncology and immunology.
- •
Significant R&D investment and global reach.
Weaknesses
Recently scaled back its infectious disease and vaccine R&D, potentially reducing direct future competition in virology.
Large size can sometimes lead to slower pivots compared to more focused biotechs.
Differentiators
Breadth of portfolio across pharmaceuticals, medical devices, and consumer health.
Strong presence in multiple myeloma and prostate cancer.
Indirect Competitors
- →
Moderna / BioNTech
Description:Pioneers of mRNA technology, now applying it beyond COVID-19 vaccines to infectious diseases and oncology (e.g., cancer vaccines).
Threat Level:Medium
Potential For Direct Competition:High, especially if their mRNA-based vaccines or therapies for HIV or cancer prove successful.
- →
Innovative Biotech Firms
Description:Smaller, agile biotech companies focused on novel platforms like gene editing (CRISPR Therapeutics, Intellia Therapeutics) or new cell therapy approaches that could disrupt current treatment paradigms.
Threat Level:Low
Potential For Direct Competition:Medium, as they are often acquisition targets for larger players like Gilead, but could emerge as competitors if their technology matures independently.
- →
Generic and Biosimilar Manufacturers
Description:Companies like Teva, Viatris, and Sandoz that produce low-cost generic versions of drugs once patents expire, directly impacting revenue of legacy products.
Threat Level:High (upon patent expiry)
Potential For Direct Competition:Becomes direct competition the moment a blockbuster drug loses exclusivity.
Competitive Advantage Analysis
Sustainable Advantages
- Advantage:
Market Dominance in HIV Treatment
Sustainability Assessment:Gilead has a deeply entrenched leadership position with its single-tablet regimens like Biktarvy. This advantage is sustained by a loyal prescriber base, extensive real-world data, and a robust commercial infrastructure.
Competitor Replication Difficulty:Hard
- Advantage:
Strong R&D Pipeline in Virology
Sustainability Assessment:Continuous innovation, exemplified by the development of long-acting lenacapavir, is key to managing product lifecycles and defending against competitors and patent cliffs.
Competitor Replication Difficulty:Hard
- Advantage:
Leadership in Cell Therapy via Kite Pharma
Sustainability Assessment:Gilead has a first-mover advantage and established expertise in the complex field of CAR T-cell therapy (Yescarta, Tecartus), a high-growth area with significant barriers to entry.
Competitor Replication Difficulty:Hard
- Advantage:
Extensive Patent Portfolio
Sustainability Assessment:Patents provide legal market exclusivity, a cornerstone of the biopharma business model. While individual patents expire, the overall portfolio and strategy to defend and expand it are a key advantage.
Competitor Replication Difficulty:Hard
Temporary Advantages
{'advantage': 'Exclusivity on Newly Approved Drugs', 'estimated_duration': 'Varies by patent life (typically 10-15 years post-launch). Lenacapavir (Yeztugo/Sunlenca) is the most recent example. '}
Disadvantages
- Disadvantage:
Heavy Revenue Dependence on the HIV Franchise
Impact:Critical
Addressability:Moderately. The company is actively addressing this by investing heavily in its oncology pipeline, but this diversification takes a long time and is not guaranteed to succeed.
- Disadvantage:
Looming Patent Cliffs
Impact:Critical
Addressability:Difficult. While Gilead has a strategy to manage the eventual Biktarvy patent expiration (2033), the potential revenue loss is significant and requires successful development and commercialization of new blockbusters.
- Disadvantage:
Intense Competition in Oncology
Impact:Major
Addressability:Moderately. Gilead entered a crowded and competitive space. Success depends on differentiating its assets (like Trodelvy and cell therapies) from those of established players like Merck, BMS, and Roche.
- Disadvantage:
Public Scrutiny and Litigation over Drug Pricing and Patents
Impact:Minor
Addressability:Difficult. This is an industry-wide issue that poses a continuous reputational and financial risk.
Strategic Recommendations
Quick Wins
- Recommendation:
Amplify Digital Marketing for Lenacapavir (Yeztugo) Launch
Expected Impact:High
Implementation Difficulty:Easy
- Recommendation:
Showcase Kite's Cell Therapy Patient Success Stories
Expected Impact:Medium
Implementation Difficulty:Easy
Medium Term Strategies
- Recommendation:
Accelerate Clinical Trials for Combination Therapies in Oncology
Expected Impact:High
Implementation Difficulty:Moderate
- Recommendation:
Strategic 'Bolt-On' Acquisitions of Biotechs with Mid-Stage Assets
Expected Impact:High
Implementation Difficulty:Moderate
- Recommendation:
Expand Market Access Programs for Lenacapavir in Underserved Global Markets
Expected Impact:Medium
Implementation Difficulty:Moderate
Long Term Strategies
- Recommendation:
Invest in Next-Generation Therapeutic Platforms (e.g., in-vivo cell therapy, mRNA therapies)
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Build a Leading Franchise in Inflammation
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Develop a Comprehensive Post-Biktarvy Lifecycle Strategy
Expected Impact:High
Implementation Difficulty:Difficult
Solidify position as the undisputed leader in virology while aggressively building a top-tier, differentiated oncology franchise focused on cell therapy and antibody-drug conjugates (ADCs).
Differentiate through 'transformative therapies' by focusing on first-in-class or best-in-class assets in areas of high unmet need, particularly long-acting antivirals and cutting-edge cell therapies, rather than competing on incremental improvements in crowded markets.
Whitespace Opportunities
- Opportunity:
Develop therapies for chronic viral diseases beyond HIV/Hepatitis.
Competitive Gap:While many companies focus on acute viral infections (like COVID-19 or flu), there is less competition in developing curative or suppressive therapies for chronic latent viruses.
Feasibility:Medium
Potential Impact:High
- Opportunity:
Pioneer Allogeneic ('Off-the-Shelf') CAR T-cell therapies.
Competitive Gap:Current CAR-T therapies are autologous (patient-specific), which is complex and time-consuming. A successful allogeneic platform would be a major disruptive advance.
Feasibility:Low
Potential Impact:High
- Opportunity:
Leadership in Health Equity and Access.
Competitive Gap:While many pharma companies have access programs, Gilead could build a sustainable competitive advantage by making global health equity a core, measurable part of its corporate strategy, especially with its HIV prevention drugs.
Feasibility:High
Potential Impact:Medium
- Opportunity:
Expand into novel anti-inflammatory pathways.
Competitive Gap:While the inflammation market is crowded with TNF and JAK inhibitors, there is significant opportunity for therapies with novel mechanisms of action that offer better safety or efficacy profiles.
Feasibility:Medium
Potential Impact:High
Gilead Sciences operates within a mature, oligopolistic biopharmaceutical industry characterized by exceptionally high barriers to entry. The company's competitive landscape is defined by its unparalleled dominance in the HIV market, which serves as both its greatest strength and a critical vulnerability due to high revenue concentration. Its primary sustainable competitive advantages are its market-leading HIV portfolio, a robust and innovative virology R&D pipeline, and a pioneering position in cell therapy through its Kite Pharma subsidiary.
The most intense direct competition comes from GSK's ViiV Healthcare, which is aggressively challenging Gilead's HIV franchise with alternative long-acting and 2-drug regimens. In its crucial growth area, oncology, Gilead faces formidable competition from established powerhouses like Merck, Bristol Myers Squibb, and AbbVie, making market share gains difficult and expensive. The success of its oncology portfolio, particularly Trodelvy and its CAR-T therapies, is paramount to de-risking the company from its dependency on HIV and navigating the looming patent cliff for its blockbuster, Biktarvy.
Indirect threats are emerging from companies with disruptive platform technologies, such as mRNA and gene editing, which could reshape treatment paradigms in the long term. Strategic imperatives for Gilead include flawless execution of the lenacapavir launch to extend its HIV leadership, accelerating the oncology pipeline through both internal R&D and strategic 'bolt-on' acquisitions, and making meaningful progress in its third therapeutic area, inflammation. Whitespace opportunities exist in pioneering 'off-the-shelf' cell therapies and establishing true leadership in global health equity, turning a social responsibility into a brand-defining competitive advantage.
Messaging
Message Architecture
Key Messages
- Message:
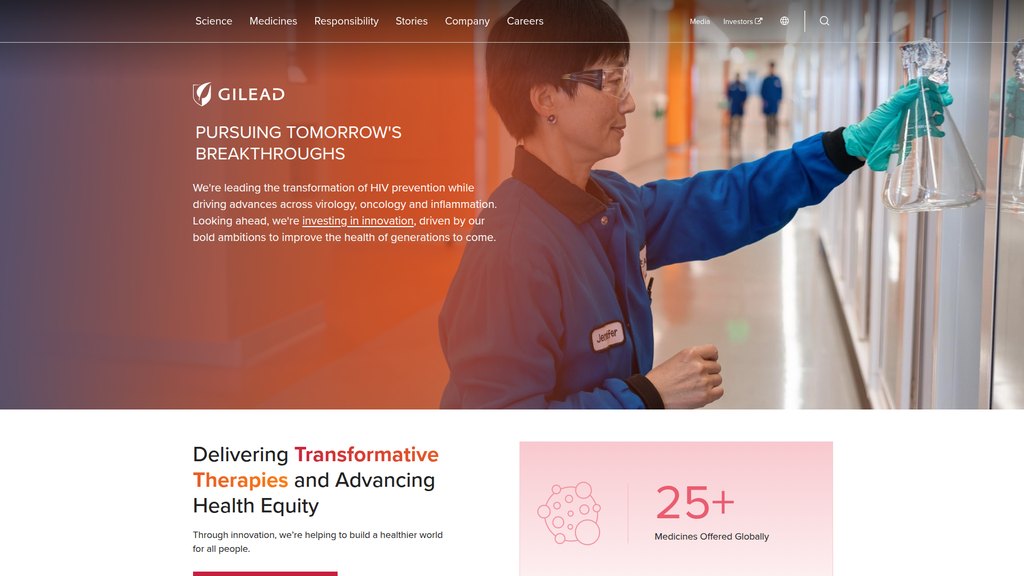
PURSUING TOMORROW'S BREAKTHROUGHS
Prominence:Primary
Clarity Score:High
Location:Homepage Hero Headline
- Message:
We're leading the transformation of HIV prevention while driving advances across virology, oncology and inflammation.
Prominence:Secondary
Clarity Score:High
Location:Homepage Hero Sub-headline
- Message:
Delivering Transformative Therapies and Advancing Health Equity
Prominence:Secondary
Clarity Score:High
Location:Homepage Mid-page Headline
- Message:
Using Science to Improve the Lives of People and Communities
Prominence:Tertiary
Clarity Score:High
Location:Homepage Content Section
- Message:
A Strong Pipeline Spanning Three Therapeutic Areas
Prominence:Tertiary
Clarity Score:High
Location:Homepage Pipeline Section
The message hierarchy is exceptionally clear and effective. The primary headline, 'PURSUING TOMORROW'S BREAKTHROUGHS,' establishes a forward-looking, innovation-focused identity. This is immediately supported by secondary messages that specify the 'what' (HIV, virology, oncology, inflammation) and the 'why' (transformative therapies, health equity). The visual design of the homepage reinforces this hierarchy, giving the most weight to the core brand promise.
Messaging is highly consistent across the provided content, including the German-language page. Core concepts like 'science', 'innovation', 'transformative therapies', and 'creating a healthier world' are woven throughout all sections, from the main headlines to the careers and science-specific areas. This creates a unified and coherent brand narrative.
Brand Voice
Voice Attributes
- Attribute:
Authoritative & Confident
Strength:Strong
Examples
- •
We're leading the transformation of HIV prevention...
- •
1 Funder of HIV Programs in the U.S.
- •
We invest in world-class science...
- Attribute:
Innovative & Forward-Looking
Strength:Strong
Examples
- •
PURSUING TOMORROW'S BREAKTHROUGHS
- •
Driving advances across virology, oncology and inflammation.
- •
Looking ahead, we're investing in innovation...
- Attribute:
Purpose-Driven & Altruistic
Strength:Moderate
Examples
- •
...driven by our bold ambitions to improve the health of generations to come.
- •
Follow our journey to create a healthier world for all people
- •
Advancing Health Equity
- Attribute:
Scientific & Professional
Strength:Strong
Examples
- •
52 Clinical Programs Across Virology, Oncology and Inflammation
- •
Using Science to Improve the Lives of People and Communities
- •
A Strong Pipeline Spanning Three Therapeutic Areas
Tone Analysis
Professional
Secondary Tones
- •
Inspirational
- •
Confident
- •
Formal
Tone Shifts
The tone becomes slightly more corporate and financially-oriented in the 'Investors' section with phrases like 'Delivering Life-Changing Therapies' linked to investor resources.
The 'People and Culture' and 'Stories@Gilead' sections adopt a softer, more community-focused tone.
Voice Consistency Rating
Excellent
Consistency Issues
No itemsValue Proposition Assessment
Gilead leverages world-class science and relentless innovation to develop and deliver transformative medicines for the world's most severe diseases, with a primary focus on virology and a growing leadership in oncology and inflammation.
Value Proposition Components
- Component:
Leadership in Virology (especially HIV)
Clarity:Clear
Uniqueness:Unique
Evidence:Explicitly stated with 'leading the transformation of HIV prevention' and the '#1 Funder' data point.
- Component:
Strong, Diversified R&D Pipeline
Clarity:Clear
Uniqueness:Somewhat Unique
Evidence:Highlighted by the '52 Clinical Programs' statistic and the prominent display of Virology, Oncology, and Inflammation as key areas.
- Component:
Commitment to Global Health Equity
Clarity:Somewhat Clear
Uniqueness:Somewhat Unique
Evidence:The phrase 'Advancing Health Equity' is used, but the 'how' is not detailed on the homepage, making it less tangible than other components.
- Component:
Foundation in Scientific Excellence
Clarity:Clear
Uniqueness:Common
Evidence:Repeated use of 'science,' 'innovation,' and 'breakthroughs' reinforces this, though it is a common claim in the biopharma industry.
Gilead's messaging successfully differentiates the company primarily through its established and heavily emphasized leadership in HIV and virology. While competitors like Pfizer, Merck, and AbbVie also focus on innovation, Gilead's specific claim as the '#1 Funder of HIV Programs' provides a concrete and powerful differentiator. The secondary message around expanding this scientific leadership into oncology and inflammation positions the company as a focused innovator rather than a generalist.
The messaging positions Gilead as a specialized, science-first leader in complex diseases. It doesn't try to compete on breadth with giants like Pfizer or J&J, but on depth and proven leadership in its core areas. The narrative is one of evolving from a dominant force in one field (virology) to a multi-faceted leader in adjacent high-need areas, positioning it as a dynamic and growing player for investors, talent, and partners.
Audience Messaging
Target Personas
- Persona:
Investors & Financial Analysts
Tailored Messages
- •
A Strong Pipeline Spanning Three Therapeutic Areas
- •
52 Clinical Programs...
- •
Driven by Purpose, Delivering Life-Changing Therapies
Effectiveness:Effective
- Persona:
Healthcare Professionals (HCPs) & Scientists
Tailored Messages
- •
PURSUING TOMORROW'S BREAKTHROUGHS
- •
We're leading the transformation of HIV prevention...
- •
View Our Pipeline
Effectiveness:Effective
- Persona:
Potential Employees (Scientific & Corporate)
Tailored Messages
- •
An Inclusive Workplace Where We All Can Make an Impact
- •
...driven by our bold ambitions to improve the health of generations to come.
- •
Explore Careers at Gilead
Effectiveness:Effective
- Persona:
Patients & General Public
Tailored Messages
- •
Delivering Transformative Therapies and Advancing Health Equity
- •
Using Science to Improve the Lives of People and Communities
- •
Follow our journey to create a healthier world for all people
Effectiveness:Somewhat
Audience Pain Points Addressed
- •
The existence of life-threatening diseases without adequate treatments (HIV, cancer, etc.).
- •
The need for continuous medical innovation to combat evolving diseases.
- •
The challenge of ensuring equitable access to advanced medical treatments.
Audience Aspirations Addressed
- •
The hope for a 'healthier world for all people.'
- •
The desire to be part of an organization that makes a meaningful impact on global health.
- •
The ambition to push the boundaries of science and medicine.
Persuasion Elements
Emotional Appeals
- Appeal Type:
Hope & Optimism
Effectiveness:High
Examples
PURSUING TOMORROW'S BREAKTHROUGHS
...improve the health of generations to come.
- Appeal Type:
Pride & Authority
Effectiveness:High
Examples
We're leading the transformation...
1 Funder of HIV Programs in the U.S.
- Appeal Type:
Purpose & Altruism
Effectiveness:Medium
Examples
Gilead Employees Give Back During Month of Service
...create a healthier world for all people.
Social Proof Elements
- Proof Type:
Data & Statistics
Impact:Strong
Evidence
- •
25+ Medicines Offered Globally
- •
52 Clinical Programs...
- •
1 Funder of HIV Programs in the U.S.
- Proof Type:
Expert Authority
Impact:Moderate
Evidence
An Open Letter from Daniel O’Day, Chairman & CEO
- Proof Type:
News & Media Coverage
Impact:Strong
Evidence
The 'News' section featuring press releases on regulatory approvals and financial results.
Trust Indicators
- •
Prominent display of quantitative data (pipeline numbers, medicines offered).
- •
Regularly updated news section with official press releases.
- •
Dedicated, easily accessible sections for Investors and Science.
- •
Clear identification of therapeutic areas of focus.
- •
Executive leadership communication (CEO's open letter).
Calls To Action
Primary Ctas
- Text:
Learn More About Gilead
Location:Homepage
Clarity:Clear
- Text:
Explore Science
Location:Homepage
Clarity:Clear
- Text:
View Our Pipeline
Location:Homepage
Clarity:Clear
- Text:
Explore Careers at Gilead
Location:Homepage
Clarity:Clear
- Text:
View Stories@Gilead
Location:Homepage
Clarity:Clear
The CTAs are clear, functional, and well-aligned with the target audience's information-seeking journeys. They effectively segment traffic towards key areas like Science, Pipeline, and Careers. However, the language is very standard and lacks persuasive power. They serve their navigational purpose well but do not actively compel or inspire action beyond simple information gathering.
Messaging Gaps Analysis
Critical Gaps
Lack of Patient Voice: The messaging is heavily company-centric ('We're leading...', 'We invest...'). There are no direct patient stories or testimonials on the homepage, which creates an emotional distance and misses an opportunity to showcase the human impact of their 'transformative therapies'.
Contradiction Points
No itemsUnderdeveloped Areas
'Health Equity' in Practice: The message 'Advancing Health Equity' is a powerful claim but remains an abstract concept on the homepage. There are no immediate examples, statistics, or stories to substantiate what this means in practice, diminishing its impact.
The 'How' Behind the Science: While the site mentions 'world-class science,' it could benefit from briefly explaining how its approach to R&D is unique or different from competitors, which is a key differentiator in a crowded market.
Messaging Quality
Strengths
- •
Exceptional clarity and consistency of the core message around science-led innovation.
- •
Powerful use of data points ('#1 Funder', '52 Clinical Programs') to substantiate claims and build authority.
- •
Strong, confident, and professional brand voice that inspires trust.
- •
Effective message hierarchy that guides the user's understanding of the company's focus and priorities.
Weaknesses
- •
Overly corporate and sterile tone that lacks a direct emotional connection with the end beneficiary: the patient.
- •
The messaging is very company-focused, speaking about Gilead's accomplishments rather than the patient lives it changes.
- •
Abstract claims like 'health equity' are not backed with tangible proof points on the homepage.
Opportunities
- •
Humanize the brand by integrating patient-centric narratives and stories that demonstrate the real-world impact of their therapies.
- •
Create a dedicated content section or module to showcase tangible 'Health Equity' initiatives, turning the claim into a compelling proof point.
- •
A/B test more benefit-oriented and persuasive CTA language to increase engagement.
Optimization Roadmap
Priority Improvements
- Area:
Homepage Narrative
Recommendation:Develop a new homepage module titled 'Transforming Lives' featuring a short, powerful patient story (video or text/image). This directly connects the 'breakthroughs' to human outcomes, bridging the current emotional gap.
Expected Impact:High
- Area:
Value Proposition
Recommendation:Expand the 'Advancing Health Equity' section with 2-3 specific proof points or a link to a dedicated page detailing programs, partnerships, and measurable impact in underserved communities.
Expected Impact:Medium
Quick Wins
Revise secondary headlines to be more patient-benefit focused. E.g., change 'Using Science to Improve the Lives of People...' to 'How Our Science is Changing Lives...'.
Test more engaging CTA copy. For example, change 'View Our Pipeline' to 'Discover Our Next Breakthrough'.
Long Term Recommendations
Conduct a comprehensive messaging audit to identify opportunities to shift from a company-centric ('we do this') to a patient-centric ('so you can do this') narrative across the entire website.
Develop a storytelling framework that consistently highlights the journey from scientific discovery to patient impact in all corporate communications, including news, stories, and reports.
Gilead's strategic messaging on its corporate website is a masterclass in establishing authority, credibility, and a clear market position. The brand voice is professional, confident, and consistently reinforces its identity as a science-first innovator with proven leadership in virology and expanding expertise in oncology and inflammation. The message architecture is logical and effective, successfully communicating a complex business model to diverse audiences like investors, scientists, and potential employees through a clear hierarchy and powerful data-driven proof points.
The primary weakness lies in its emotional resonance. The messaging is highly corporate and company-centric, speaking at length about its own actions ('pursuing,' 'leading,' 'investing') but failing to directly connect these actions to the human lives they impact. There is a significant gap where the patient voice should be; this absence creates a clinical and detached tone that undermines the emotional weight of its mission to 'create a healthier world.' While the message of 'Advancing Health Equity' is present, it is underdeveloped and lacks the tangible evidence needed to be a truly compelling differentiator.
To elevate its messaging from effective to exceptional, Gilead should focus on humanizing its brand. The current messaging successfully sells the company; the opportunity now is to tell the story of the lives it changes. By weaving in patient-centric narratives and providing concrete evidence for its social commitments, Gilead can bridge the gap between its scientific authority and its human purpose, creating a more powerful, persuasive, and differentiated brand identity in the competitive biopharmaceutical landscape.
Growth Readiness
Growth Foundation
Product Market Fit
Strong
Evidence
- •
Dominant market share (over 50%) in the global HIV treatment market, a core area of focus.
- •
Biktarvy, the flagship HIV treatment, is a blockbuster drug with sales growing 13% to $13.4 billion in 2024.
- •
Robust pipeline with 52 clinical programs, indicating significant investment in future product-market fit across Virology, Oncology, and Inflammation.
- •
Successful expansion into oncology with rapidly growing revenues from Trodelvy and cell therapies (Yescarta, Tecartus), demonstrating ability to establish fit in new therapeutic areas.
- •
Strong global presence and regulatory success, evidenced by recent European Commission authorization for Lenacapavir for HIV prevention.
Improvement Areas
- •
Reducing revenue concentration from the HIV franchise, which still accounts for the majority of sales.
- •
Accelerating the commercial success of the oncology portfolio to achieve the strategic goal of one-third of revenue from oncology by 2030.
- •
Strengthening the inflammation pipeline, which has historically faced setbacks, to create a third powerful revenue pillar.
Market Dynamics
Approximately 8.2% to 8.8% CAGR for the global biopharmaceutical market through 2030-2033.
Mature
Market Trends
- Trend:
Shift towards biologics, cell and gene therapies.
Business Impact:High. Gilead's acquisitions of Kite Pharma (CAR-T) and Interius BioTherapeutics (in vivo platform) position it well to capitalize on this trend. Continued investment is critical for leadership.
- Trend:
Increasing focus on oncology and immunology.
Business Impact:High. Aligns with Gilead's strategic pivot. Success in these highly competitive areas is crucial for future growth as oncology is the largest and fastest-growing segment of the biopharma market.
- Trend:
Patent cliffs and the rise of biosimilars/generics.
Business Impact:Critical. Gilead faces a significant patent cliff for its top-seller Biktarvy in 2033. The strategy to launch new HIV treatments like long-acting Lenacapavir is a direct and necessary response to mitigate this future revenue loss.
- Trend:
Intensifying pricing pressure and reimbursement hurdles from payers.
Business Impact:Medium. As a major player, Gilead faces constant pricing negotiations. Demonstrating superior clinical value and developing innovative therapies are key to maintaining pricing power.
- Trend:
Use of AI in drug discovery to shorten development timelines.
Business Impact:Medium. An emerging opportunity to accelerate R&D. Competitors are adopting AI, and Gilead must invest to maintain R&D efficiency.
Favorable. While the market is mature, Gilead is well-timed to capitalize on high-growth segments like cell therapy and long-acting antivirals. Its strategic M&A and pipeline focus align with major industry growth vectors.
Business Model Scalability
High
Characterized by extremely high, fixed R&D and clinical trial costs, but relatively low variable costs for manufacturing small molecule drugs once approved, leading to high gross margins (often 85%+). Biologics and cell therapies have higher variable costs but still offer significant operating leverage.
High. Once a drug's development costs are covered, each additional sale contributes significantly to profit, enabling rapid profit scaling with successful commercialization.
Scalability Constraints
- •
Regulatory approval timelines and complexities across different geographies.
- •
Manufacturing capacity and complexity, especially for cell therapies which are highly personalized.
- •
Securing market access and reimbursement from a diverse and fragmented global payer landscape.
- •
The finite lifecycle of products due to patent expirations.
Team Readiness
Strong. Experienced leadership with a track record of executing large-scale M&A (Kite, Immunomedics, CymaBay) and navigating complex global product launches.
Complex global matrix structure typical of large pharma. Appears effective for managing distinct therapeutic areas but may pose challenges for cross-functional agility and rapid integration of acquisitions.
Key Capability Gaps
- •
In Vivo Gene Editing & Delivery: The recent acquisition of Interius BioTherapeutics suggests a recognized need to build deeper capabilities in this next-generation therapeutic modality.
- •
Digital & AI in R&D: While likely investing, accelerating the integration of AI and machine learning into drug discovery and clinical trial optimization is a key competitive frontier.
- •
Commercialization in Highly Competitive Oncology Markets: While growing, Gilead is still challenging established leaders like Merck and Bristol Myers Squibb, requiring sustained investment in commercial talent and strategy.
Growth Engine
Acquisition Channels
- Channel:
Medical Affairs & Scientific Engagement (MSLs, Conferences, Publications)
Effectiveness:High
Optimization Potential:Medium
Recommendation:Increase focus on digital opinion leader engagement and virtual scientific congresses to broaden reach and data dissemination beyond traditional in-person events.
- Channel:
Sales Force & Direct-to-Physician Marketing
Effectiveness:High
Optimization Potential:Medium
Recommendation:Leverage data analytics to optimize sales force deployment and personalize physician engagement based on prescribing patterns and patient demographics.
- Channel:
Payer & Health System Negotiations (Market Access)
Effectiveness:High
Optimization Potential:High
Recommendation:Develop more sophisticated value-based pricing and outcomes-based contracts with payers to accelerate formulary access for high-cost therapies like cell therapy and new long-acting agents.
- Channel:
Public Health & Advocacy Group Partnerships
Effectiveness:High
Optimization Potential:Medium
Recommendation:Expand partnerships to support disease awareness and screening initiatives, particularly for HIV prevention (PrEP) and viral hepatitis, to grow the addressable patient population.
Customer Journey
The 'customer' journey involves multiple stakeholders: Physician awareness -> Clinical conviction -> Payer reimbursement -> Prescription -> Patient adherence. It is a long, complex, data-driven process.
Friction Points
- •
Restrictive drug formularies or prior authorization requirements from insurers.
- •
Competition for physician attention in crowded therapeutic areas like oncology.
- •
Logistical complexity of administering advanced therapies like CAR-T (e.g., patient cell collection, manufacturing).
- •
Patient adherence to chronic therapies.
Journey Enhancement Priorities
- Area:
Payer Access
Recommendation:Invest in generating robust real-world evidence (RWE) to supplement clinical trial data, better demonstrating long-term value and cost-effectiveness to payers.
- Area:
Physician Education
Recommendation:Develop advanced digital tools and educational platforms to help physicians navigate complex treatment decisions, especially for cell therapies and combination regimens in oncology.
- Area:
Patient Support
Recommendation:Enhance patient services programs to improve onboarding, financial assistance, and adherence, particularly for new long-acting injectable therapies.
Retention Mechanisms
- Mechanism:
Superior Clinical Profile (Efficacy & Safety)
Effectiveness:High
Improvement Opportunity:Continue investing in head-to-head clinical trials to clearly establish superiority over competitors, defending market share for key products like Biktarvy.
- Mechanism:
Long-Acting Formulations
Effectiveness:High
Improvement Opportunity:Successfully launch and drive adoption of twice-yearly Lenacapavir for HIV prevention and treatment, a significant retention and adherence driver that locks in patients for longer periods.
- Mechanism:
Patient Support Programs
Effectiveness:Medium
Improvement Opportunity:Integrate digital health tools (apps, reminders) into support programs to provide more proactive and personalized adherence support.
Revenue Economics
Extremely Favorable for Commercialized Drugs. The biopharma model involves massive upfront R&D investment (billions) and high risk of failure. However, for an approved, patented drug, gross margins are exceptionally high (80-90%+), and the lifetime value of a patient on a chronic therapy is substantial.
Not a standard metric. A better proxy is 'Portfolio Return on R&D Investment'. This is strong, driven by the massive success of the HIV and HCV franchises, which have funded diversification.
High. The company generates significant revenue ($28.8B in 2024) from a focused portfolio of high-value products.
Optimization Recommendations
- •
Secure broad market access and favorable pricing for new launches (e.g., Lenacapavir) early in the product lifecycle.
- •
Pursue label expansion for existing drugs (e.g., Trodelvy in new cancer types) to maximize the return on initial R&D investment.
- •
Improve manufacturing efficiency for complex biologics and cell therapies to reduce cost of goods sold (COGS).
Scale Barriers
Technical Limitations
- Limitation:
Manufacturing complexity and scalability of CAR-T cell therapies.
Impact:High
Solution Approach:Invest in manufacturing innovation and process automation. The acquisition of an 'in vivo' CAR-T platform (Interius) is a strategic move to potentially circumvent the complex 'ex vivo' process entirely.
- Limitation:
Scientific risk in the R&D pipeline (clinical trial failures).
Impact:High
Solution Approach:Maintain a diversified pipeline across different therapeutic areas and modalities. Utilize a portfolio approach, balancing high-risk/high-reward early-stage assets with lower-risk late-stage programs and M&A.
Operational Bottlenecks
- Bottleneck:
Global supply chain management for biologics and temperature-sensitive drugs.
Growth Impact:Potential for launch delays or supply interruptions.
Resolution Strategy:Build redundant and geographically diverse supply chains. Invest in advanced logistics and cold-chain technologies.
- Bottleneck:
Integrating large-scale acquisitions (people, processes, pipelines).
Growth Impact:Failure to realize synergies and potential disruption to ongoing operations.
Resolution Strategy:Establish a dedicated, experienced M&A integration office with a standardized playbook to ensure smooth transitions and value capture.
Market Penetration Challenges
- Challenge:
Patent expirations for key revenue drivers, notably Biktarvy in 2033.
Severity:Critical
Mitigation Strategy:Execute a lifecycle management strategy by launching next-generation therapies with superior profiles (e.g., long-acting Lenacapavir) to convert patients before the patent cliff.
- Challenge:
Intense competition in oncology from established players with broad portfolios (Merck, BMS, Roche).
Severity:Major
Mitigation Strategy:Focus on areas of high unmet need and differentiated mechanisms of action. Pursue strategic combinations of proprietary drugs and invest heavily in clinical development to prove superiority.
- Challenge:
Gaining market access and favorable reimbursement from global payers.
Severity:Major
Mitigation Strategy:Engage payers early in the development process and build robust health economics and outcomes research (HEOR) data packages to justify the value and price of new therapies.
Resource Limitations
Talent Gaps
- •
Leading scientists in next-generation modalities (e.g., in vivo gene editing, targeted protein degradation).
- •
Data scientists and AI/ML specialists for drug discovery and clinical trial analytics.
- •
Experts in global market access and value-based contracting.
High and continuous. Requires billions annually for internal R&D, plus a multi-billion dollar capacity for strategic, bolt-on, and potentially large-scale M&A to acquire new technologies and late-stage assets.
Infrastructure Needs
Expansion of specialized manufacturing facilities for cell and gene therapies.
Investment in a robust data infrastructure to support AI-driven R&D and commercial operations.
Growth Opportunities
Market Expansion
- Expansion Vector:
Geographic expansion in emerging markets (e.g., China, Latin America).
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Establish strategic partnerships with local players to navigate regulatory hurdles and build commercial infrastructure. Prioritize markets with growing healthcare spending and large patient populations.
- Expansion Vector:
Demographic: Expanding HIV PrEP market.
Potential Impact:High
Implementation Complexity:Medium
Recommended Approach:Leverage the convenience of twice-yearly Lenacapavir to significantly grow the HIV prevention market beyond current high-risk populations through targeted awareness campaigns and public health partnerships.
Product Opportunities
- Opportunity:
Advance the Oncology Pipeline, particularly cell therapy (anito-cel) and ADCs (Trodelvy).
Market Demand Evidence:Oncology is the largest and fastest-growing therapeutic area in biopharma. Cell therapy market for multiple myeloma is estimated to reach $12B by 2030.
Strategic Fit:High. Aligns with the core strategy of diversifying revenue beyond HIV.
Development Recommendation:Aggressively fund late-stage clinical trials for anito-cel to target a 2026 launch. Continue exploring new indications for Trodelvy through combination studies.
- Opportunity:
Develop In Vivo Cell Therapies via the newly acquired Interius platform.
Market Demand Evidence:Significant pharma interest and investment in in vivo approaches to overcome the logistical and cost challenges of ex vivo CAR-T.
Strategic Fit:High. Represents the next frontier of cell therapy and a major long-term competitive advantage.
Development Recommendation:Establish the Philadelphia site as a center of excellence, fully funding the platform's development to move from oncology into autoimmune diseases.
- Opportunity:
Expand the Inflammation portfolio through M&A and licensing.
Market Demand Evidence:Large, multi-billion dollar markets in diseases like rheumatoid arthritis, Crohn's disease, and lupus.
Strategic Fit:Medium-High. A stated third pillar of the company's strategy, but the current pipeline is less mature than virology and oncology.
Development Recommendation:Pursue bolt-on acquisitions or licensing deals for mid-to-late stage inflammation assets to accelerate pipeline development, similar to the CymaBay acquisition in liver disease.
Channel Diversification
- Channel:
Digital Health Partnerships
Fit Assessment:High
Implementation Strategy:Partner with digital health companies to create 'beyond the pill' solutions that improve patient adherence, monitor outcomes, and generate real-world data to support value propositions to payers.
- Channel:
Academic & Research Institution Collaborations
Fit Assessment:High
Implementation Strategy:Expand early-stage research collaborations to access novel targets and technologies, creating an external innovation engine that complements internal R&D efforts.
Strategic Partnerships
- Partnership Type:
Technology Platform Licensing/Acquisition
Potential Partners
AI-driven drug discovery companies (e.g., Recursion, Exscientia)
Novel drug delivery platform companies (e.g., for oral biologics, targeted delivery)
Expected Benefits:Accelerate early-stage pipeline development, shorten discovery timelines, and enhance the efficacy and delivery of future therapies.
- Partnership Type:
Co-development & Co-commercialization
Potential Partners
Biotech companies with promising mid-stage assets in oncology or inflammation
Other large pharma companies for combination trials (e.g., existing partnership with Merck)
Expected Benefits:Share the high cost and risk of late-stage development while accessing complementary assets for powerful combination therapies.
Growth Strategy
North Star Metric
Pipeline Value Progression & Diversification
Financial results are a lagging indicator of past R&D success. The most critical driver of long-term growth is the successful advancement of high-value assets through the clinical pipeline, particularly in non-HIV areas, to secure future revenue streams and reduce concentration risk.
Achieve 3-5 new late-stage (Phase 3 or registration) trial initiations per year, with at least 60% focused on oncology and inflammation.
Growth Model
Science-Led M&A and Commercialization
Key Drivers
- •
Internal R&D success in virology and oncology.
- •
Strategic acquisitions of innovative platforms and late-stage assets (e.g., Kite, Immunomedics, Interius).
- •
Best-in-class clinical development and regulatory execution.
- •
Effective global commercial launches and market access.
A dual-pronged approach: 1) Protect and extend the virology cash cow through next-generation products. 2) Aggressively reinvest cash flow into building leadership positions in oncology and inflammation through a disciplined 'string of pearls' M&A strategy.
Prioritized Initiatives
- Initiative:
Diversify Revenue by Accelerating the Oncology Franchise
Expected Impact:High
Implementation Effort:High
Timeframe:3-5 years
First Steps:Fully fund Phase 3 trials for anito-cel targeting a 2026 launch. Initiate new combination studies for Trodelvy to expand its addressable market.
- Initiative:
Win the Next Era of HIV with Long-Acting Lenacapavir
Expected Impact:High
Implementation Effort:Medium
Timeframe:1-3 years
First Steps:Execute a flawless commercial launch for the PrEP indication, focusing on securing broad payer coverage and funding patient/physician education on the benefits of a twice-yearly injectable.
- Initiative:
Build the Next Therapeutic Platform with In Vivo Cell Therapy
Expected Impact:High (Long-term)
Implementation Effort:High
Timeframe:5-10 years
First Steps:Successfully integrate the Interius BioTherapeutics acquisition and establish a clear R&D roadmap for the first clinical candidates in both oncology and autoimmune disease.
Experimentation Plan
High Leverage Tests
- Experiment:
Basket trials for Trodelvy across multiple tumor types with a specific biomarker.
Hypothesis:We can rapidly identify new, high-potential indications for label expansion.
- Experiment:
Real-world evidence study comparing adherence and outcomes of long-acting Lenacapavir vs. daily oral PrEP.
Hypothesis:Demonstrating superior real-world adherence and effectiveness will justify premium pricing and drive rapid adoption.
- Experiment:
Pilot value-based contracts with a major payer for Yescarta (CAR-T therapy).
Hypothesis:Tying payment to patient outcomes can accelerate market access and establish a new commercial model for high-cost curative therapies.
Clinical trial endpoints (e.g., Overall Survival, Response Rate), Real-World Adherence Rates, Time-to-Formulary-Access, and Return on R&D Investment.
Clinical development follows multi-year phases. The 'cadence' is governed by a rigorous portfolio review process, typically on a quarterly and annual basis, to make 'go/no-go' decisions on pipeline assets.
Growth Team
A cross-functional 'Therapeutic Area Leadership Team' model for each key area (Virology, Oncology, Inflammation). These teams should be empowered with decision-making authority across R&D, business development, and commercial functions.
Key Roles
- •
Head of Business Development / Search & Evaluation (to identify M&A targets)
- •
Head of Clinical Development Operations
- •
Global Market Access Lead
- •
Head of Data Science & AI (reporting into R&D leadership)
Continue aggressive M&A to acquire new technological capabilities. Establish an internal 'Gilead Ventures' arm to make early-stage investments in promising biotech startups, providing a window into emerging science.
Gilead Sciences demonstrates a strong foundation for growth, anchored by its dominant and highly profitable HIV franchise. However, the company faces a critical strategic inflection point due to the eventual patent expiration of its blockbuster drug, Biktarvy. The leadership team has correctly identified this challenge and is executing a clear, multi-pronged strategy to drive future growth and diversify its revenue base.
The primary growth engine is a strategic pivot to oncology, which is showing significant momentum with strong sales growth from Trodelvy and its CAR-T cell therapies. The ambition to derive one-third of revenue from this segment by 2030 is aggressive but achievable if the pipeline, including promising assets like anito-cel, delivers. This diversification is the most critical element of the long-term growth story.
The second key growth vector is the reinvention and extension of the core HIV franchise through innovation. The development of long-acting injectables like Lenacapavir is a masterful lifecycle management strategy, poised to not only defend against generic competition but also significantly expand the HIV prevention (PrEP) market by addressing the key challenge of daily pill adherence.
Growth is being further catalyzed by a disciplined, science-led M&A strategy. Recent acquisitions like CymaBay (liver disease), and particularly Interius BioTherapeutics (in vivo cell therapy), are not just filling pipeline gaps but are strategic acquisitions of next-generation technology platforms that can provide a sustainable competitive advantage for the next decade.
Key barriers remain, including intense competition in oncology, the inherent risks of clinical development, and increasing pricing pressures from global payers. Success will hinge on flawless execution in three areas: 1) Accelerating the oncology pipeline to market, 2) Ensuring the successful global launch and adoption of Lenacapavir, and 3) Effectively integrating new technology platforms to build the next wave of therapeutic innovation. Overall, Gilead is in a strong position, transitioning from a company defined by virology to a more diversified biopharmaceutical leader.
Legal Compliance
Gilead maintains a comprehensive and easily accessible set of privacy statements. They have a main Privacy Statement and specific policies for different user groups like Healthcare Professionals (HCPs) and job applicants, demonstrating a mature approach to data governance. The policy details the types of personal information collected (both directly and through automated means like cookies), the purposes for collection (e.g., providing services, clinical research, drug safety), and methods for data collection. Importantly, it states that Gilead does not sell personal information to third parties. For international data transfers, Gilead notes it relies on appropriate safeguards like Standard Contractual Clauses for data moving from the EU/Switzerland to the U.S., which is crucial for GDPR compliance. The policy provides clear contact methods (web portal, email, phone, mail) for users to exercise their privacy rights, such as access or deletion requests. The presence of a separate 'Consumer Health Data Privacy Policy' indicates proactive steps to comply with emerging U.S. state-level health data laws.
The Terms of Use are robust and typical for a high-risk industry. Key strengths include a clear disclaimer of warranties and limitation of liability, stating that information is provided 'AS IS' and should not be considered medical advice. This is a critical risk mitigation strategy. The terms explicitly protect Gilead's intellectual property (copyrights and trademarks) while granting users a personal, non-commercial license to use the site content. The policy also includes a clause disclaiming responsibility for third-party websites linked from their site. For certain international versions of the site, there are audience-specific restrictions, such as limiting access to verified Healthcare Professionals in the UK, which is an excellent control to prevent off-label promotion to the general public. However, the clause stating that any user-transmitted communication is on a 'non-confidential basis' and can be used for any purpose could create user trust issues, even if it is legally protective.
Gilead provides a detailed Cookie Statement that categorizes cookies into Strictly Necessary, Performance, Functional, Targeting, and Social Media. This granular classification is a best practice under GDPR and other modern privacy laws. The website implements a cookie consent banner that links to a 'Manage Cookies Settings' portal, allowing users to make choices about different cookie categories. This demonstrates a clear mechanism for obtaining user consent. The policy explains the function of each cookie type and names specific technologies used, such as Google Analytics, providing transparency. It also correctly notes that in some jurisdictions, performance cookies may be sent before banner acceptance, a detail that reflects a nuanced understanding of varying legal requirements.
Gilead's data protection framework appears sophisticated, addressing both GDPR for its European operations and U.S. privacy laws. The Privacy Statement explicitly provides web portals for residents of the EEA and the US to submit data rights requests, indicating operational readiness to handle Data Subject Access Requests (DSARs). Their withdrawal from the Privacy Shield framework and reliance on Standard Contractual Clauses (SCCs) for EU-U.S. data transfers is a legally sound and up-to-date approach. The statement that they do not 'sell' personal information is a key disclosure for CCPA/CPRA compliance. The separate privacy notices for different data subjects (e.g., HCPs) show a mature, risk-based approach to data protection, recognizing that different data types and subjects carry different legal obligations.
While the website footer on some regional pages links to an 'Accessibility Statement', a comprehensive, centrally located statement on the main gilead.com site was not immediately apparent from the scraped data and initial search. The presence of such statements on regional sites is positive, but a global commitment is a best practice. Pharmaceutical companies, whose audiences often include individuals with health challenges, have a heightened ethical and legal imperative to ensure accessibility. The FDA and other bodies recommend conformance with WCAG 2.0 Level AA or higher. Without a clear, global statement and evidence of features like sufficient color contrast, ARIA labels for dynamic content, and full keyboard navigability, this stands as a potential area of risk and improvement.
As a biopharmaceutical company, Gilead's website operates under stringent regulations from the FDA, EMA, and other global health authorities.
- Drug Promotion & Advertising: The website effectively uses disclaimers to manage regulatory risk, such as the notice 'Some of the content on this page is not intended for users outside the U.S.'. This is a crucial strategy to avoid violating international prohibitions on direct-to-consumer advertising for prescription drugs. The content focuses on corporate information, scientific innovation, and pipeline, rather than making specific product claims, which helps mitigate the risk of 'off-label' promotion. Regional sites for healthcare professionals require verification, which is a key control.
- SEC Regulations: The 'Investors' section and SEC filings appropriately contain detailed 'Forward-Looking Statements' disclaimers. These safe harbor statements are legally required by the Private Securities Litigation Reform Act of 1995 and are essential for managing investor liability.
- Physician Payments Sunshine Act (Open Payments): Gilead's website provides information regarding its compliance with the Sunshine Act, which mandates the public reporting of payments and transfers of value to physicians and teaching hospitals. This transparency is a legal requirement in the U.S. and demonstrates adherence to industry-wide ethical standards.
- PhRMA Code: The company's interactions and communications appear to align with the principles of the PhRMA Code on Interactions with Health Care Professionals, which governs ethical relationships and is crucial for maintaining trust and avoiding perceptions of impropriety.
Compliance Gaps
- •
Lack of a prominent, easily accessible global Accessibility Statement (WCAG/ADA compliance) on the main corporate homepage.
- •
The Terms of Use clause claiming the right to use user-submitted information on a non-confidential basis for any purpose is legally protective but could undermine user trust and may conflict with privacy expectations under GDPR.
- •
Absence of explicit information on a designated Data Protection Officer (DPO) in the main privacy policy, which is a specific requirement under GDPR for many organizations.
Compliance Strengths
- •
Robust and granular privacy policies tailored to different user groups (e.g., HCPs, consumers).
- •
Clear and effective use of geo-targeting disclaimers to manage differing international regulations on drug promotion.
- •
Strong SEC compliance with clear 'Forward-Looking Statements' disclaimers in investor-facing materials.
- •
Implementation of a granular cookie consent mechanism that allows users to opt-in/out of specific cookie categories.
- •
Clear mechanisms for users in different jurisdictions (EEA, US) to exercise their data privacy rights.
- •
Transparent approach to Sunshine Act reporting and adherence to the PhRMA code of conduct.
Risk Assessment
- Risk Area:
Regulatory Enforcement (FDA/EMA)
Severity:High
Recommendation:Maintain and continuously audit the clear separation between corporate/investor information and product-specific promotional content. Ensure all content is reviewed for 'fair balance' and avoids any hint of off-label promotion. Strengthen geo-fencing and audience-gating mechanisms for country-specific content.
- Risk Area:
Data Privacy Litigation (GDPR/CCPA)
Severity:Medium
Recommendation:Appoint and explicitly name a Data Protection Officer (DPO) in the privacy policy to fully align with GDPR requirements. Review the 'non-confidential' clause in the Terms of Use to ensure it doesn't overreach and conflict with GDPR's principles of purpose limitation and data minimization.
- Risk Area:
Accessibility Lawsuits (ADA)
Severity:Medium
Recommendation:Conduct a formal accessibility audit against WCAG 2.1 AA standards. Publish a global Accessibility Statement on gilead.com that details the company's commitment, conformance level, and provides a channel for users with disabilities to report issues. This is a significant area of litigation risk in the U.S.
- Risk Area:
Securities Litigation (SEC)
Severity:Low
Recommendation:Continue the current best practice of including detailed forward-looking statement disclaimers in all financial communications, press releases, and investor relations content. Ensure these disclaimers are regularly updated to reflect current risk factors.
High Priority Recommendations
- •
Commission an external audit of the global website (gilead.com) for compliance with Web Content Accessibility Guidelines (WCAG) 2.1 Level AA.
- •
Publish a comprehensive, global Accessibility Statement on the main website's footer, affirming commitment to accessibility and providing a feedback mechanism.
- •
Update the Privacy Statement to explicitly name a Data Protection Officer (DPO) and their contact information to ensure full compliance with GDPR Article 37.
- •
Review and potentially revise the 'non-confidential communication' clause in the Terms of Use to better align with user privacy expectations and the principle of purpose limitation under GDPR.
Gilead Sciences demonstrates a sophisticated and mature legal compliance posture, which is a significant strategic asset in the highly regulated biopharmaceutical industry. The company's digital presence is clearly structured to mitigate the highest-severity risks, particularly those associated with off-label drug promotion and securities law. The use of geographic disclaimers and audience-gating for healthcare professionals are best-in-class examples of using legal compliance to enable market access while managing risk. Their data privacy framework is robust, with clear policies and mechanisms that address the complexities of GDPR and CCPA, fostering trust with patients, healthcare providers, and partners. This strong foundation supports business model scalability across different legal jurisdictions.
The primary area for strategic improvement is in digital accessibility. In an industry focused on health, ensuring the website is fully usable by people with disabilities is not only a matter of legal risk (ADA lawsuits are common and costly) but also a core component of corporate social responsibility and brand trust. Addressing this gap would further solidify Gilead's position as a leader in ethical and compliant business practices. Minor refinements to the Terms of Use and explicit designation of a DPO would further harden their already strong data protection framework against regulatory scrutiny.
Visual
Design System
Modern Corporate
Excellent
Advanced
User Experience
Navigation
Horizontal Top Navigation Bar (Mega Menu likely on hover)
Intuitive
Good
Information Architecture
Logical
Clear
Moderate
Conversion Elements
- Element:
Hero Section CTA ('Creating Possible')
Prominence:Medium
Effectiveness:Somewhat effective
Improvement:The primary headline is a brand statement, not a call-to-action. While visually appealing, a more direct value proposition or action-oriented subheading could improve immediate user engagement.
- Element:
Section CTA Buttons (e.g., 'View Our Pipeline', 'Explore Stories')
Prominence:Medium
Effectiveness:Effective
Improvement:The ghost button style (outline only) has lower visual weight than the filled-in buttons. Standardize the primary CTA style to the filled, high-contrast magenta button for greater consistency and to more clearly guide the user.
- Element:
News Section Links ('View News')
Prominence:Low
Effectiveness:Ineffective
Improvement:The 'View News' link is small and easily missed. Elevate this to a standard button-style CTA to encourage deeper engagement with corporate communications, which is critical for investor and media audiences.
- Element:
Footer Links
Prominence:Low
Effectiveness:Effective
Improvement:The footer is well-organized and serves its purpose for secondary navigation. The hierarchy between 'Quick Links', 'Get in Touch', and 'Legal' is clear.
Assessment
Strengths
- Aspect:
Strong Brand Identity & Visual Cohesion
Impact:High
Description:The website effectively uses Gilead's brand color (a vibrant magenta/red) as a consistent accent for all interactive elements like buttons and links. This, combined with high-quality, authentic photography, creates a professional, trustworthy, and unified brand experience.
- Aspect:
Clear Information Architecture
Impact:High
Description:The homepage is logically structured into clear, thematic sections (e.g., 'Therapeutic Areas', 'Scientific Innovation', 'Workplace', 'News'). This allows diverse audiences (HCPs, investors, potential employees) to quickly find relevant information pathways.
- Aspect:
Effective Use of Human-Centric Imagery
Impact:Medium
Description:The photography focuses on diverse groups of people—scientists in labs and diverse corporate teams. This visual storytelling effectively communicates Gilead's mission of scientific innovation and its commitment to an inclusive culture, humanizing the corporate brand.
Weaknesses
- Aspect:
Inconsistent CTA Design
Impact:Medium
Description:There is a mix of filled buttons, ghost buttons (outline), and simple text links with arrows for calls-to-action. This inconsistency can dilute the visual hierarchy and confuse the user about which actions are most important.
- Aspect:
Low Prominence of Key Statistics
Impact:Medium
Description:The key metrics ('25+ Medicines', '52 Clinical Programs', '#1 in HIV') are presented in a visually muted, light-gray box. These are powerful proof points that could be given more visual weight and color to increase their impact, especially for investor and partner audiences.
- Aspect:
Dense Text in Some Modules
Impact:Low
Description:Some descriptive text paragraphs, such as under 'Delivering Transformative Health Equity', are center-aligned and wide, which can decrease readability. Left-aligning text for longer paragraphs is generally better for scannability.
Priority Recommendations
- Recommendation:
Standardize All Primary and Secondary CTAs
Effort Level:Low
Impact Potential:High
Rationale:Create a clear visual distinction between primary (filled magenta button) and secondary (outline or text link with arrow) calls-to-action. Applying this system consistently across the site will create a more intuitive user path, improve conversion on key goals, and strengthen the design system.
- Recommendation:
Enhance the Visual Impact of Key Data Points
Effort Level:Low
Impact Potential:Medium
Rationale:Redesign the statistics module to be more visually engaging. Incorporate the brand's primary color or use stronger typography to make these impressive numbers stand out. This will increase the perception of Gilead as a market leader and innovator.
- Recommendation:
Optimize Readability of Body Copy
Effort Level:Low
Impact Potential:Low
Rationale:Adjust typography settings for body text. Specifically, left-align paragraphs of text longer than two lines and ensure the line width does not exceed an optimal character count (typically 50-75 characters) to reduce cognitive load and improve readability.
Mobile Responsiveness
Good
The component-based, card-style design suggests a layout that will adapt well to various breakpoints. The full-width image and content blocks will likely stack vertically in a clean manner.
Mobile Specific Issues
- •
The three-column statistics block will need to stack into a single column to remain legible. This could make the section quite long on mobile.
- •
The main navigation will collapse into a 'hamburger' menu; its internal organization and usability will be critical for the mobile experience.
- •
The news section with three cards will stack vertically, which is standard and effective.
Desktop Specific Issues
The maximum width of the content on large desktop monitors appears well-constrained, preventing text lines from becoming too long and unreadable, which is a common best practice.
Gilead's website presents a mature, professional, and visually cohesive digital presence that effectively communicates its identity as a leading biopharmaceutical company. The design system is advanced, marked by excellent brand consistency through the strategic use of color, high-quality photography, and clean typography. This projects an image of credibility and innovation.
The information architecture is a key strength. The homepage is clearly segmented to serve the distinct needs of its diverse audiences, including Healthcare Professionals, investors, patients, and prospective employees. The user flow from broad brand messaging to specific content areas like 'Pipeline' or 'Careers' is logical and well-signposted. The use of authentic, human-centric imagery successfully bridges the gap between a large corporation and its human impact, reinforcing its mission to create a healthier world.
However, there are clear opportunities for conversion optimization and UX refinement. The most significant weakness is the inconsistent application of call-to-action (CTA) styles. The mix of filled buttons, ghost buttons, and text links lacks a clear hierarchy, potentially confusing users and reducing engagement with key content funnels. Key data points, which serve as powerful social proof, are visually underplayed and could be highlighted more effectively to bolster brand perception.
From a strategic perspective, the immediate priority should be to standardize the CTA design system. This low-effort, high-impact change will create a more intuitive user journey. Following that, enhancing the visual prominence of key statistics and refining body text readability will further elevate the user experience, ensuring the site not only looks professional but also performs optimally in guiding users to critical information.
Discoverability
Market Visibility Assessment
Gilead has established formidable brand authority, particularly in virology (HIV), where it is a market leader. Its digital presence effectively communicates its legacy and commitment to ending the HIV epidemic. However, its authority in oncology and inflammation is still developing and less prominent in search visibility compared to established competitors like Bristol Myers Squibb and Roche. The corporate website serves as a primary channel for disseminating pivotal scientific data and clinical trial results, reinforcing its reputation among healthcare professionals (HCPs) and investors.
Gilead's digital visibility mirrors its market dominance in HIV, with its products like Biktarvy commanding significant search interest among clinicians. However, in the increasingly competitive long-acting HIV prevention space, competitors like GSK's ViiV Healthcare are building significant digital momentum and share of voice. In oncology, Gilead's visibility is growing with assets like Trodelvy but does not yet challenge the entrenched digital presence of competitors like Merck (Keytruda) or BMS. The digital 'shelf space' for oncology and inflammation terms is highly contested, indicating an opportunity for Gilead to increase its share of voice.
For a biopharmaceutical company, 'customer acquisition' translates to engaging Healthcare Professionals (HCPs), attracting top scientific talent, and securing investor confidence. The website's clear segmentation for 'Science,' 'Investors,' and 'Careers' directly supports these distinct acquisition funnels. The high potential lies in leveraging its deep scientific content (pipeline, clinical programs) to organically attract and engage KOLs and prescribing physicians searching for cutting-edge therapeutic information. The 'Stories@Gilead' section provides a platform for employer branding to attract talent, but this could be more strategically integrated with scientific content to attract R&D professionals.
The website shows a clear strategy for global market penetration, evidenced by multilingual content (e.g., the German sub-site) and news releases detailing approvals from international bodies like the European Commission. This signals a digital strategy that supports global commercial operations. There is a significant opportunity to create more localized content hubs for key markets, addressing specific regional healthcare challenges, regulatory environments, and patient advocacy group priorities, which would deepen market penetration beyond simple translation.
Gilead's content squarely focuses on its three pillars: Virology, Oncology, and Inflammation. The coverage in virology is exceptionally deep, reflecting its market leadership. Coverage in oncology is robust, detailing the pipeline and key products. However, the content is heavily weighted towards corporate announcements and clinical data. There is a strategic opportunity to broaden topic coverage to include disease state education, health equity initiatives, and the broader impact of their research, which would capture a wider audience earlier in their information-seeking journey and build broader topical authority.
Strategic Content Positioning
The content is well-aligned with the later stages of the professional customer journey (e.g., HCPs evaluating clinical data, investors reviewing financial results). The detailed 'Pipeline' and 'Investor Relations' sections serve these audiences effectively. However, it is less aligned with the early 'awareness' and 'education' stages. An HCP or researcher exploring a new therapeutic mechanism, rather than a specific Gilead product, may not find sufficient non-promotional, educational content to engage with, representing a gap at the top of the engagement funnel.
Gilead's CEO letters and 'Stories' provide a foundation for thought leadership. The primary opportunity is to expand beyond a corporate-centric narrative by creating dedicated, high-value content platforms for each therapeutic area. This could involve featuring external Key Opinion Leaders (KOLs), hosting scientific webinars, and publishing in-depth analyses of market trends like the future of cell therapy or long-acting antivirals. Positioning their internal scientists as accessible experts would further solidify their image as an innovation powerhouse.
Competitors like GSK/ViiV are highly focused on the narrative around HIV prevention and patient access. While Gilead is a leader in this space, its digital content could more forcefully articulate its vision and commitment to health equity to counter competitor messaging. In oncology, where many competitors have strong patient-facing (where regulations allow) and HCP educational resources, Gilead has an opportunity to build out more comprehensive disease-state educational hubs around the cancers its therapies target, thereby capturing search interest beyond branded product queries.
The core brand message of 'Pursuing Tomorrow's Breakthroughs' and 'Creating a healthier world' is consistently applied across the corporate sections of the site. This message is reinforced by press releases and stories about scientific innovation. The messaging is professional, scientifically grounded, and consistent with that of a leading biopharmaceutical firm. The challenge is ensuring this high-level message translates into engaging and accessible content for diverse audiences beyond the investment community.
Digital Market Strategy
Market Expansion Opportunities
- •
Develop comprehensive, unbranded digital resource centers for oncology and inflammatory diseases to build authority and capture market interest in these growth areas.
- •
Create localized content strategies for key international markets (e.g., Japan, EU5) that address specific healthcare system needs and KOL interests, moving beyond direct translation.
- •
Launch a dedicated digital platform focused on the future of virology, encompassing emerging viruses and next-generation treatments, to solidify and expand their leadership position beyond HIV.
Customer Acquisition Optimization
- •
Invest in creating high-value, gated content for HCPs (e.g., CME-accredited webinars, deep-dive mechanism-of-action videos) to generate qualified leads for medical science liaison (MSL) engagement.
- •
Develop a robust 'Science and Innovation' content hub that profiles Gilead's researchers and their work, serving as a powerful tool for attracting top-tier scientific and R&D talent.
- •
Optimize scientific and clinical data for search visibility to ensure HCPs and researchers find Gilead's resources first when searching for information on relevant compounds and trial results.
Brand Authority Initiatives
- •
Launch a 'Gilead Innovators' series featuring interviews and articles with internal and external scientific leaders to personify the company's commitment to R&D.
- •
Systematically publish and promote lay-friendly summaries of their scientific publications and conference presentations to increase media citation and public understanding.
- •
Establish a digital advisory board of leading KOLs to co-create content and guidance, lending third-party credibility to Gilead's thought leadership platforms.
Competitive Positioning Improvements
- •
Intensify digital communications around health equity and patient access programs, particularly for the HIV portfolio, to counter strong narratives from competitors like ViiV Healthcare.
- •
Create a superior digital experience for oncologists by providing practical resources, such as clinical trial finders and data visualization tools, differentiating Gilead through utility and support.
- •
Amplify the narrative around the Kite acquisition and cell therapy leadership to build a distinct and forward-looking brand identity in the highly competitive oncology market.
Business Impact Assessment
Success is measured not by direct sales, but by digital 'share of voice' for key therapeutic area keywords (e.g., 'long-acting HIV PrEP', 'CAR T-cell therapy advances'). Tracking rankings and visibility against competitors like GSK, BMS, and Merck for these non-branded terms serves as a leading indicator of market influence.
Key metrics include engagement rates from HCPs on clinical data pages, downloads of scientific publications, registrations for professional webinars, and the volume of qualified applicants to R&D positions citing digital content as a source. These demonstrate effective engagement with target professional audiences.
Authority is measured by the quality and quantity of backlinks from reputable medical institutions, academic journals, and top-tier media outlets. An increase in organic search traffic to the 'Science' and 'Pipeline' sections, coupled with rising media mentions of Gilead's research, would signify growing brand authority.
Benchmarking involves regular analysis of competitor digital strategies. This includes comparing content depth in oncology, share of voice in virology discussions on professional networks, and the sentiment of media coverage regarding innovation pipelines. The goal is to lead, not lag, key competitors in digital presence within strategic therapeutic areas.
Strategic Recommendations
High Impact Initiatives
- Initiative:
Launch Therapeutic Area Command Centers: Develop dedicated, content-rich digital hubs for Virology, Oncology, and Inflammation, moving beyond simple landing pages.
Business Impact:High
Market Opportunity:Establishes Gilead as the definitive source of information in its core and growth areas, capturing significant organic traffic from HCPs and researchers, and building authority against entrenched competitors.
Success Metrics
- •
Organic search rankings for non-branded therapeutic keywords
- •
Time on site and engagement rate within hubs
- •
Inbound inquiries from KOLs and researchers
- •
Share of voice against key competitors
- Initiative:
Create a 'KOL Digital Engagement Program': Systematically identify and collaborate with digital opinion leaders (DOLs) and established KOLs to co-create and amplify scientific content.
Business Impact:High
Market Opportunity:Leverages trusted, independent voices to validate and disseminate Gilead's scientific narrative, building credibility faster and more effectively than branded content alone.
Success Metrics
- •
Reach and engagement of co-created content
- •
Sentiment analysis of online KOL conversations
- •
Referral traffic from KOL blogs and social platforms
- Initiative:
Develop a 'Health Equity and Access' Content Pillar: Create a dedicated section of the site showcasing initiatives, partnerships, and data related to improving access to medicines globally.
Business Impact:Medium
Market Opportunity:Strengthens corporate reputation, enhances ESG narratives for investors, and builds trust with patient advocacy groups and policymakers, which is a key competitive factor in the HIV space.
Success Metrics
- •
Media mentions of health equity initiatives
- •
Engagement from advocacy and policy-related organizations
- •
Inclusion in ESG and corporate responsibility reports
Evolve Gilead's digital presence from a corporate broadcast channel into a dynamic, multi-stakeholder scientific engagement platform. The strategy is to leverage its profound scientific depth to not only showcase its products but to own the broader scientific conversations in its therapeutic areas. By becoming an indispensable digital resource for HCPs, researchers, and talent, Gilead can translate its market leadership in virology into burgeoning authority in oncology and inflammation, creating a durable competitive advantage built on trust and expertise.
Competitive Advantage Opportunities
- •
Leverage the decades of trust and relationships built in the HIV community to create a 'halo effect' of credibility for its newer oncology and inflammation portfolios.
- •
Build the industry's leading digital resource for CAR T-cell therapy, providing unparalleled educational content and support for clinicians navigating this complex and transformative treatment modality.
- •
Pioneer a new standard for transparency and data visualization around its clinical pipeline, making complex information accessible and engaging for both specialist and investor audiences.
Gilead Sciences possesses a strong and authoritative digital presence that accurately reflects its corporate stature and market leadership, particularly in virology. The current website, gilead.com, functions effectively as a central repository for corporate communications, scientific data, and investor relations, serving its primary professional audiences with detailed, credible information.
However, the digital landscape in the biopharmaceutical industry is shifting from static information delivery to dynamic scientific engagement. While Gilead excels at communicating its achievements, its primary strategic opportunity lies in evolving its digital platform to lead the scientific discourse in its chosen fields. The company's main competitors, such as GSK/ViiV in HIV and established giants like BMS and Merck in oncology, are aggressively competing for the attention of healthcare professionals and key opinion leaders online.
To secure future market leadership and build a defensible competitive moat, Gilead must transition its digital strategy from a 'portfolio of products' to a 'platform for progress.' This involves expanding content beyond its own pipeline to cover the broader therapeutic landscapes of virology, oncology, and inflammation. By creating comprehensive, non-promotional educational resources, Gilead can capture the interest of its target audiences earlier in their discovery process, building trust and establishing itself as an indispensable partner in advancing patient care.
Key strategic initiatives should focus on creating dedicated therapeutic area 'Command Centers' to consolidate expertise, launching a formal digital KOL program to amplify its scientific narrative through trusted voices, and more forcefully communicating its significant work in health equity. These actions will not only enhance its brand authority and support its commercial objectives but will also create a powerful engine for attracting the next generation of scientific talent essential for its long-term mission. The ultimate goal is for Gilead's digital presence to be as innovative and transformative as its science.
Strategic Priorities
Strategic Priorities
- Title:
Accelerate Oncology Franchise to Critical Mass
Business Rationale:Directly addresses the primary strategic risk of revenue over-concentration on the HIV franchise ahead of the 2033 Biktarvy patent cliff. Success in the high-growth oncology market is the most critical component of the company's long-term diversification and growth strategy.
Strategic Impact:Transforms Gilead from a virology-dominant company into a diversified biopharmaceutical leader, significantly de-risking the business model and creating a second major growth engine capable of driving long-term enterprise value.
Success Metrics
- •
Achieve strategic goal of >33% total revenue from oncology by 2030
- •
Market share growth for Trodelvy, Yescarta, and Tecartus in their key indications
- •
Successful Phase 3 data readout and regulatory submission for anito-cel
Priority Level:HIGH
Timeline:Strategic Initiative
Category:Market Position
- Title:
Establish Market Dominance in Long-Acting Virology
Business Rationale:The core HIV franchise funds the company's entire diversification effort. It is imperative to protect this cash-flow engine by leading the market's evolution from daily oral pills to long-acting injectables, thereby extending franchise longevity and defending against intense competition from GSK/ViiV.
Strategic Impact:Reshapes the HIV treatment and prevention market around Gilead's next-generation technology (Lenacapavir), creating a new, durable revenue stream that bridges the company through the Biktarvy patent cliff and reinforces its scientific leadership.
Success Metrics
- •
Annual revenue from Lenacapavir-based regimens
- •
Patient conversion rate from oral therapies to long-acting injectables
- •
Market share of the long-acting HIV prevention (PrEP) segment
Priority Level:HIGH
Timeline:Strategic Initiative
Category:Revenue Model
- Title:
Operationalize Next-Generation Therapeutic Platforms
Business Rationale:Long-term leadership depends on owning the next wave of therapeutic innovation. The acquisition of platforms for in-vivo cell therapy (Interius) is a strategic bet to solve the cost and complexity barriers of current cell therapies, creating a highly defensible, long-term competitive advantage.
Strategic Impact:Positions Gilead to leapfrog competitors by pioneering scalable, 'off-the-shelf' cell therapies. This transforms the Kite subsidiary from a leader in today's cell therapy to the defining force in tomorrow's, with applications potentially expanding beyond oncology to autoimmune diseases.
Success Metrics
- •
Successful integration of the Interius R&D platform
- •
Achievement of preclinical and IND-filing milestones for the first in-vivo candidate
- •
Establishment of new strategic partnerships to leverage the platform
Priority Level:HIGH
Timeline:Long-term Vision
Category:Operations
- Title:
Shift Brand Narrative from 'Science-First' to 'Patient-Impact'
Business Rationale:Analysis reveals a brand voice that is authoritative but overly corporate and emotionally distant. In increasingly competitive markets like oncology, a brand narrative centered on the human impact of its science is a powerful differentiator that builds trust with patients, providers, and payers.
Strategic Impact:Evolves the brand from a competent drug manufacturer to a purpose-driven life sciences leader. This humanized positioning strengthens stakeholder relationships, enhances corporate reputation, and can improve market access and adoption of new therapies.
Success Metrics
- •
Year-over-year improvement in brand perception surveys (measuring patient-centricity and trust)
- •
Increased media share of voice around patient impact stories vs. purely scientific data
- •
Higher engagement rates on patient-focused digital content
Priority Level:MEDIUM
Timeline:Strategic Initiative
Category:Brand Strategy
- Title:
Weaponize 'Health Equity' as a Competitive Differentiator
Business Rationale:Gilead's messaging around 'Health Equity' is currently an abstract claim. Operationalizing this concept through concrete, scaled-up global access programs for new prevention therapies (like Lenacapavir) can create a profound competitive advantage, particularly in the HIV market.
Strategic Impact:Establishes Gilead as the definitive global leader in ending the HIV epidemic, moving beyond scientific leadership to societal impact. This strengthens its social license to operate, provides a powerful ESG narrative for investors, and builds a brand halo that competitors cannot easily replicate.
Success Metrics
- •
Number of individuals on Lenacapavir for PrEP in low- and middle-income countries (LMICs)
- •
Successful establishment of large-scale public-private partnerships for access
- •
Measurable reduction in HIV incidence rates in target regions
Priority Level:MEDIUM
Timeline:Strategic Initiative
Category:Market Position
Gilead's immediate imperative is to accelerate its transformation into a diversified biopharma leader by aggressively scaling its oncology franchise to mitigate its critical dependency on HIV revenue. This must be achieved while simultaneously fortifying its virology leadership through the market-shaping launch of next-generation long-acting therapies.
The key competitive advantage to build is leadership in 'transformative therapeutic platforms,' evolving from a product-centric model to one based on defensible, next-generation technologies like advanced cell therapy (Kite) and in-vivo gene editing.
The primary growth catalyst is the successful clinical and commercial execution of the oncology pipeline, converting deep R&D investment into market-leading products that can rival the scale of the existing HIV franchise.