eScore
incyte.comThe eScore is a comprehensive evaluation of a business's online presence and effectiveness. It analyzes multiple factors including digital presence, brand communication, conversion optimization, and competitive advantage.
Incyte demonstrates a strong digital presence with a professional, well-structured corporate website and a clear global-local domain strategy. The content is well-aligned with its core audiences (HCPs, patients, investors), showing good search intent alignment. Content authority is high within its niche, evidenced by its scientific focus and consistent brand messaging, though it faces stiff competition for broader disease-state keywords from larger pharmaceutical companies.
Excellent segmentation of the website for distinct global audiences and user journeys (Patients, HCPs, Investors), which is critical for compliance and effective communication.
Develop comprehensive, unbranded 'disease education hubs' to capture significant top-of-funnel search traffic for conditions like vitiligo and myelofibrosis, establishing authority before a brand is introduced.
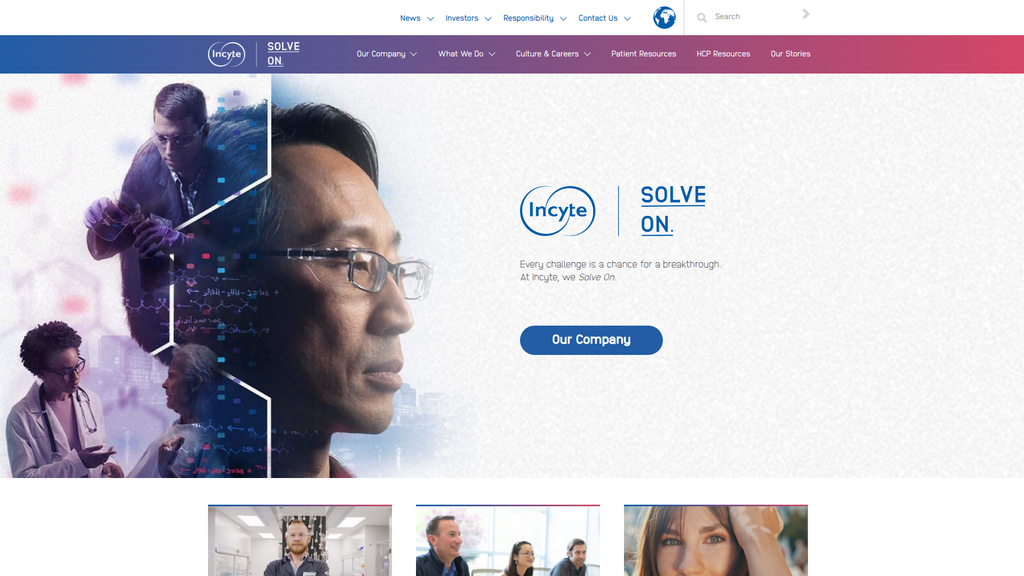
The brand communication is exceptionally disciplined and effective, anchored by the powerful and memorable 'Solve On.' mantra. This central theme is woven consistently throughout the site, effectively framing the company's identity around resilience and scientific perseverance. Messaging is clearly segmented for different audiences and powerfully uses emotional appeals of hope and purpose, backed by strong corporate trust indicators.
The 'Solve On.' tagline serves as a powerful, memorable, and consistent brand anchor that successfully differentiates the company's ethos from larger, more diffuse competitors.
More explicitly connect the high-level 'Solve On.' brand promise to the specific, tangible benefits of commercialized products on the homepage to immediately showcase the human impact of the mission.
For a biopharma company, 'conversion' means guiding specific audiences to critical information. Incyte's clear information architecture and audience-specific navigation paths are effective at this. However, the user experience is slightly hindered by understated interactive feedback (e.g., subtle link hover states), which can create minor friction. The homepage also lacks direct, prominent calls-to-action for its key patient and HCP audiences, requiring them to navigate through menus.
A clean, professional aesthetic and logical information architecture create a low cognitive load, allowing users to easily navigate to complex information.
Introduce dedicated, prominent CTA blocks on the homepage for Patients ('Find Support') and HCPs ('Access Clinical Data') to significantly shorten user journeys to critical resources.
Incyte exhibits a mature and robust approach to credibility and risk management, crucial for its industry. The website features a comprehensive and transparent legal compliance framework, including a sophisticated cookie consent mechanism and detailed privacy policies for global regulations (GDPR, CCPA). Credibility is further bolstered by extensive third-party validation through clinical trial data, regulatory approvals (FDA, EMA), and a clear separation of investor and medical information.
A robust, granular, and transparent legal compliance framework, particularly the clear segmentation of content for different audiences to meet pharmaceutical marketing regulations.
Publish a dedicated, visible accessibility statement detailing the level of WCAG conformance the site aims to achieve to formalize its commitment and mitigate potential legal risk under the ADA.
Incyte's competitive advantage is strong but concentrated. It has a defensible moat built on deep scientific expertise in JAK inhibition and first-mover advantages with Jakafi (myelofibrosis) and Opzelura (vitiligo). However, this strength is tempered by a high revenue dependency on the ruxolitinib franchise and the impending patent cliff for Jakafi, alongside intensifying competition from larger pharmaceutical players.
Deep, defensible scientific expertise in the JAK pathway, which has produced multiple first-in-class or best-in-class therapies and provides a strong foundation for pipeline development.
Accelerate pipeline diversification and pursue strategic acquisitions to build new advantages and mitigate the critical risk of revenue concentration on the soon-to-expire Jakafi patent.
The company has extremely high scalability potential, characteristic of the biopharmaceutical model with high fixed R&D costs and high-margin, scalable product revenue post-approval. Incyte has a proven global commercialization engine and a robust late-stage pipeline, signaling strong readiness for expansion. Growth is primarily contingent on continued R&D success and navigating global regulatory and reimbursement hurdles.
A proven and efficient R&D and commercialization engine that has successfully launched blockbuster drugs and is now poised to advance a diversified late-stage pipeline.
Strategically invest in building expertise and capabilities in next-generation therapeutic modalities (e.g., biologics, cell therapies) to ensure the R&D engine remains competitive for the long term.
Incyte's business model is highly coherent and proven. It effectively executes the classic R&D-driven biopharma strategy: leveraging deep scientific expertise to address unmet medical needs, protecting innovations with intellectual property, and monetizing through high-margin specialty drugs. The company's strategic focus is clear, and its resource allocation (high R&D spend) directly supports its core value proposition of scientific leadership.
Excellent alignment between its value proposition (solving unmet needs), key activities (high R&D investment), and revenue model (premium-priced, patent-protected drugs).
Address the strategic threat of revenue concentration by using its strong cash flow to aggressively pursue M&A, ensuring the business model remains coherent post-Jakafi patent expiration.
Incyte holds significant market power as the established leader in the MPN space with Jakafi and as a first-mover in vitiligo with Opzelura. This has afforded it considerable pricing power for these innovative drugs. However, its overall market power is constrained by its smaller scale compared to giant competitors like AbbVie and Pfizer, who have greater resources and broader portfolios, creating intense pressure in core markets.
Market leadership and pricing power in niche indications (myelofibrosis, vitiligo) where it was the first to market with a highly effective therapy.
Diversify the portfolio to reduce customer dependency on the ruxolitinib franchise, which will strengthen negotiating leverage with payers and reduce overall market risk.
Business Overview
Business Classification
Biopharmaceutical
Research & Development (R&D) Intensive
Pharmaceuticals
Sub Verticals
- •
Oncology
- •
Dermatology
- •
Inflammation & Autoimmunity
- •
Hematology
Mature
Maturity Indicators
- •
Globally commercialized blockbuster drug (Jakafi/Jakavi)
- •
Established global sales and distribution networks
- •
Consistent revenue growth from product sales and royalties
- •
Robust and diversified clinical pipeline across multiple phases
- •
Active in strategic acquisitions and partnerships
Enterprise
Steady
Revenue Model
Primary Revenue Streams
- Stream Name:
Product Sales
Description:Direct sales of proprietary, commercialized drugs to specialty pharmacies and wholesalers. This is led by the blockbuster JAK inhibitor Jakafi (ruxolitinib) for myelofibrosis and polycythemia vera, and the rapidly growing topical cream Opzelura (ruxolitinib) for atopic dermatitis and vitiligo.
Estimated Importance:Primary
Customer Segment:Healthcare Providers (via distributors)
Estimated Margin:High
- Stream Name:
Royalties & Licensing Fees
Description:Revenue generated from out-licensing drug development and commercialization rights to partners in specific geographies or indications. A prime example is the collaboration with Novartis for the ex-U.S. rights to ruxolitinib (marketed as Jakavi) and with Eli Lilly for baricitinib (marketed as Olumiant).
Estimated Importance:Secondary
Customer Segment:Pharmaceutical Partners (e.g., Novartis, Eli Lilly)
Estimated Margin:High
Recurring Revenue Components
Chronic therapy drug sales (e.g., Jakafi for myelofibrosis)
Long-term royalty streams from partnered products
Pricing Strategy
Value-Based Pricing
Premium
Opaque
Pricing Psychology
Prestige pricing based on innovation and clinical efficacy
Tiered pricing based on indications and geography (via partners)
Monetization Assessment
Strengths
- •
Strong market exclusivity for key products backed by patents.
- •
High-margin revenue from both direct sales and royalties.
- •
Successful commercialization of Jakafi, achieving blockbuster status.
- •
Rapid revenue growth from Opzelura, diversifying the portfolio.
Weaknesses
Significant revenue concentration on a single product, Jakafi, creating risk.
Impending patent cliff for Jakafi around 2028, which will expose it to generic competition.
Opportunities
- •
Label expansion for existing drugs like Opzelura into new indications (e.g., hidradenitis suppurativa).
- •
Successful commercialization of late-stage pipeline candidates to offset future Jakafi revenue loss.
- •
Geographic expansion into new markets for approved products.
Threats
- •
Generic or biosimilar competition post-patent expiration.
- •
Increased competition from other novel therapies in oncology and dermatology.
- •
Pricing pressure from payers and governments.
- •
Clinical trial failures or regulatory setbacks for pipeline candidates.
Market Positioning
Scientific Leadership in Targeted Therapies
Leader in JAK Inhibition
Target Segments
- Segment Name:
Oncology & Hematology Specialists
Description:Physicians treating patients with myeloproliferative neoplasms (MPNs), graft-versus-host disease (GVHD), and other hematological cancers.
Demographic Factors
Board-certified Hematologists/Oncologists
Practicing in hospitals and specialized cancer centers
Psychographic Factors
- •
Value clinical data and efficacy
- •
Seek innovative treatments for high unmet need populations
- •
Influenced by peer-reviewed publications and medical conferences
Behavioral Factors
Prescribe targeted therapies
Manage complex patient cases requiring long-term treatment
Pain Points
- •
Limited effective treatment options for rare blood cancers
- •
Managing severe side effects of traditional therapies
- •
Controlling debilitating symptoms that impact patient quality of life
Fit Assessment:Excellent
Segment Potential:Medium
- Segment Name:
Dermatologists
Description:Physicians treating patients with chronic inflammatory skin conditions like atopic dermatitis (eczema) and autoimmune disorders like vitiligo.
Demographic Factors
Board-certified Dermatologists
Operating in private practice or academic medical centers
Psychographic Factors
- •
Seek safe and effective long-term treatment options
- •
Value novel mechanisms of action beyond traditional steroids
- •
Focus on improving patient quality of life and visible symptoms
Behavioral Factors
Prescribe both topical and systemic therapies
Early adopters of new, approved treatments
Pain Points
- •
Lack of effective treatments for repigmentation in vitiligo.
- •
Need for non-steroidal options for chronic atopic dermatitis.
- •
Patient dissatisfaction with existing therapies.
Fit Assessment:Excellent
Segment Potential:High
- Segment Name:
Patients with Chronic Conditions
Description:Individuals diagnosed with myelofibrosis, polycythemia vera, atopic dermatitis, or vitiligo seeking effective, long-term treatments.
Pain Points
- •
Debilitating symptoms impacting daily life
- •
Psychosocial burden of visible skin diseases (vitiligo, atopic dermatitis)
- •
Lack of curative therapies for their condition
Fit Assessment:Good
Segment Potential:High
Market Differentiation
- Factor:
Pioneering JAK Pathway Expertise
Strength:Strong
Sustainability:Sustainable
- Factor:
First-in-Class Approvals
Strength:Strong
Sustainability:Temporary
- Factor:
Robust Clinical Pipeline
Strength:Moderate
Sustainability:Sustainable
Value Proposition
Discovering, developing, and commercializing innovative medicines to solve serious unmet medical needs in oncology and inflammation.
Excellent
Key Benefits
- Benefit:
Provides effective treatment for rare blood cancers with limited options.
Importance:Critical
Differentiation:Unique
Proof Elements
Jakafi's FDA approval for myelofibrosis and polycythemia vera
Extensive clinical trial data published in major medical journals
- Benefit:
Offers the first and only FDA-approved treatment for repigmentation in vitiligo.
Importance:Critical
Differentiation:Unique
Proof Elements
Opzelura's FDA approval for nonsegmental vitiligo
Patient testimonials and before/after photos
- Benefit:
Delivers a non-steroidal topical option for chronic atopic dermatitis.
Importance:Important
Differentiation:Somewhat unique
Proof Elements
Opzelura's FDA approval for atopic dermatitis
Safety and efficacy data from clinical studies
Unique Selling Points
- Usp:
Deep scientific expertise and leadership in the Janus kinase (JAK) inhibitor class of drugs.
Sustainability:Long-term
Defensibility:Strong
- Usp:
A diversified pipeline targeting novel pathways in oncology and immunology.
Sustainability:Long-term
Defensibility:Moderate
Customer Problems Solved
- Problem:
Lack of effective therapies for myeloproliferative neoplasms (MPNs).
Severity:Critical
Solution Effectiveness:Complete
- Problem:
No approved treatments to restore skin color for vitiligo patients.
Severity:Major
Solution Effectiveness:Partial
- Problem:
Need for safe, long-term, non-steroidal treatments for atopic dermatitis.
Severity:Major
Solution Effectiveness:Complete
Value Alignment Assessment
High
Incyte targets diseases with high unmet needs, where innovation commands premium pricing and rapid adoption by the medical community.
High
The company's focus on groundbreaking science and clinical data directly addresses the core needs of specialist physicians and their patients seeking better outcomes.
Strategic Assessment
Business Model Canvas
Key Partners
- •
Novartis (Jakavi ex-U.S. commercialization)
- •
Eli Lilly (Olumiant development and commercialization)
- •
MorphoSys (Monjuvi development and commercialization)
- •
Agenus, MacroGenics (Antibody development collaborations)
- •
Genesis Therapeutics (AI-driven drug discovery)
- •
Academic and research institutions
Key Activities
- •
Drug discovery and scientific research
- •
Conducting pre-clinical and clinical trials (Phase I-IV)
- •
Navigating global regulatory approval processes (FDA, EMA, etc.)
- •
Pharmaceutical manufacturing and supply chain management
- •
Marketing and sales to healthcare professionals
Key Resources
- •
Intellectual Property (patents on ruxolitinib and other compounds)
- •
World-class scientific and clinical development talent
- •
Robust R&D pipeline of drug candidates
- •
Global commercialization infrastructure and sales force
- •
Strong balance sheet and cash flow for reinvestment
Cost Structure
- •
Research & Development (R&D) expenses, representing the largest cost.
- •
Selling, General & Administrative (SG&A) expenses for marketing and sales.
- •
Cost of Goods Sold (COGS) for manufacturing
- •
Milestone payments and royalties for in-licensed compounds
- •
Costs associated with acquisitions and collaborations
Swot Analysis
Strengths
- •
Market leadership and strong brand recognition with Jakafi in the MPN space.
- •
Successful launch and rapid growth of Opzelura, diversifying revenue base.
- •
Proven R&D capabilities in small molecule drug discovery, particularly JAK inhibitors.
- •
Profitable operations with strong cash flow to fund pipeline development.
Weaknesses
- •
High revenue dependency on the ruxolitinib franchise (Jakafi and Opzelura).
- •
Past clinical trial failures and pipeline setbacks.
- •
Relatively smaller scale compared to large pharma competitors like AbbVie and Novartis.
Opportunities
- •
Advance the next wave of pipeline assets to commercialization to prepare for the Jakafi patent cliff.
- •
Expand into new therapeutic areas through strategic acquisitions (e.g., Escient Pharmaceuticals).
- •
Leverage AI and new technologies to accelerate drug discovery.
- •
Further international expansion for Opzelura and other pipeline drugs.
Threats
- •
Loss of market exclusivity for Jakafi expected around 2028-2029.
- •
Intensifying competition in both oncology and dermatology from large and small pharma.
- •
Stringent regulatory environment and potential for drug pricing reforms.
- •
The inherent risk of failure in late-stage clinical trials.
Recommendations
Priority Improvements
- Area:
Pipeline Acceleration & De-risking
Recommendation:Aggressively advance late-stage (Phase 3) assets with the highest probability of success to ensure new revenue streams are established well before the Jakafi patent expiration. Prioritize povorcitinib and other high-potential programs.
Expected Impact:High
- Area:
Revenue Diversification
Recommendation:Continue strategic business development and M&A to acquire mid-to-late stage assets in core therapeutic areas (oncology, dermatology) to further reduce reliance on the ruxolitinib franchise.
Expected Impact:High
- Area:
Opzelura Market Penetration
Recommendation:Focus on maximizing Opzelura's market share by expanding payer coverage, executing on label expansions (e.g., pediatric atopic dermatitis), and increasing patient/physician education on its unique benefits for vitiligo.
Expected Impact:Medium
Business Model Innovation
Expand technology platforms beyond small molecules into biologics (monoclonal antibodies) and potentially cell therapies, through partnerships or acquisitions, to address a wider range of biological targets.
Develop a more integrated patient support services model, using digital health tools to improve treatment adherence and gather real-world evidence, thereby strengthening the value proposition to payers.
Revenue Diversification
Acquire a commercial-stage asset in an adjacent therapeutic area to add an immediate, non-correlated revenue stream.
Establish more co-development/co-commercialization partnerships for early-to-mid stage assets to share R&D costs and risks while retaining significant upside potential.
Incyte represents a highly successful, R&D-driven biopharmaceutical company that has effectively translated deep scientific expertise in the JAK pathway into a multi-billion dollar commercial enterprise. Its business model is centered on the classic pharmaceutical strategy: high-risk, high-reward innovation protected by intellectual property. The company's flagship product, Jakafi, is a blockbuster that provides the financial foundation for its extensive pipeline. The recent success of Opzelura demonstrates Incyte's ability to execute commercially and has initiated a crucial diversification of its revenue base.
The primary strategic challenge confronting Incyte is the looming patent expiration of Jakafi around 2028. The company's future valuation and growth trajectory are critically dependent on its ability to successfully navigate this patent cliff. This requires flawless execution in advancing its late-stage pipeline, maximizing the lifecycle of its current products through label and geographic expansion, and pursuing astute strategic acquisitions to bolster its portfolio. The current business model is robust and well-suited for the industry, but its sustainability hinges entirely on continued R&D productivity and successful commercialization of new assets to replace the inevitable decline of its lead product.
Competitors
Competitive Landscape
Mature
Moderately concentrated
Barriers To Entry
- Barrier:
High Research & Development (R&D) Costs
Impact:High
- Barrier:
Stringent Regulatory Approval Process (FDA, EMA)
Impact:High
- Barrier:
Intellectual Property & Patent Protection
Impact:High
- Barrier:
Complex Manufacturing and Supply Chain
Impact:Medium
- Barrier:
Market Access and Payer Reimbursement
Impact:Medium
Industry Trends
- Trend:
Rise of JAK Inhibitors in Autoimmune Diseases
Impact On Business:Positive, as Incyte is a leader with Jakafi and Opzelura, but also increases direct competition from other companies developing JAK inhibitors.
Timeline:Immediate
- Trend:
Focus on Precision Oncology
Impact On Business:Aligned with Incyte's strategy of developing targeted therapies for specific genetic mutations in cancers.
Timeline:Immediate
- Trend:
Growth in Dermatology Market for Inflammatory Conditions
Impact On Business:Significant growth driver for Opzelura in atopic dermatitis and vitiligo.
Timeline:Immediate
- Trend:
Increased Scrutiny on Drug Pricing and Reimbursement
Impact On Business:Potential pressure on revenue and profitability for high-cost specialty drugs like Jakafi.
Timeline:Near-term
- Trend:
Emergence of Novel Therapeutic Modalities (e.g., Cell & Gene Therapy)
Impact On Business:Potential long-term threat as alternative treatment approaches could displace small molecule drugs in certain indications.
Timeline:Long-term
Direct Competitors
- →
AbbVie Inc.
Market Share Estimate:Leader in immunology with significant overlap in dermatology.
Target Audience Overlap:High
Competitive Positioning:Global biopharmaceutical giant with a broad portfolio in immunology and a powerful commercial infrastructure.
Strengths
- •
Blockbuster drug Rinvoq (upadacitinib), a direct JAK inhibitor competitor, approved for multiple autoimmune indications including atopic dermatitis.
- •
Extensive global marketing and sales capabilities.
- •
Strong pipeline in immunology and oncology.
- •
Significant financial resources for R&D and acquisitions.
Weaknesses
Faces patent cliffs for major products like Humira.
JAK inhibitor class-wide safety concerns (black box warnings) could impact market adoption of Rinvoq.
Differentiators
Broad portfolio of both small molecules and biologics.
Aggressive clinical development program for Rinvoq across numerous indications.
- →
Pfizer Inc.
Market Share Estimate:Major player in inflammation and immunology.
Target Audience Overlap:High
Competitive Positioning:Global pharmaceutical leader with a diverse portfolio and a strong focus on JAK inhibitors for inflammatory diseases.
Strengths
- •
Multiple JAK inhibitors, including Xeljanz (tofacitinib) and Cibinqo (abrocitinib), competing in similar therapeutic areas.
- •
Cibinqo directly competes with Opzelura in atopic dermatitis.
- •
Vast global commercial and manufacturing footprint.
- •
Strong brand recognition among physicians and patients.
Weaknesses
The first-generation status of Xeljanz comes with more established safety concerns, which has led to increased scrutiny of the entire JAK class.
Faces competition from more selective next-generation JAK inhibitors.
Differentiators
Portfolio of multiple JAK inhibitors targeting different specificities.
Deep experience in launching and marketing blockbuster drugs globally.
- →
Bristol Myers Squibb
Market Share Estimate:Significant competitor in hematology/oncology.
Target Audience Overlap:Medium
Competitive Positioning:Leading global biopharmaceutical company with a strong focus on innovative cancer therapies.
Strengths
- •
Owns Inrebic (fedratinib), a JAK inhibitor that directly competes with Incyte's Jakafi in myelofibrosis.
- •
Strong portfolio of oncology drugs.
- •
Well-established relationships with oncologists and hematologists.
Weaknesses
Inrebic has a black box warning for serious and fatal encephalopathy, potentially limiting its use compared to Jakafi.
Less focused on dermatology compared to Incyte's expanding portfolio.
Differentiators
Broad oncology pipeline with multiple modalities (immunotherapy, targeted therapy).
Strong presence in both solid tumors and hematologic malignancies.
- →
Vertex Pharmaceuticals
Market Share Estimate:Leader in cystic fibrosis, emerging in other specialty areas.
Target Audience Overlap:Low
Competitive Positioning:Science-driven company focused on transformative medicines for serious diseases, particularly those with a clear genetic link.
Strengths
- •
Dominant market position in cystic fibrosis provides substantial revenue for R&D.
- •
Strong pipeline in specialty diseases like sickle cell disease, pain, and kidney diseases.
- •
Reputation for groundbreaking scientific innovation.
Weaknesses
High dependence on the cystic fibrosis franchise.
Limited commercial experience in oncology or dermatology.
Differentiators
Focus on curative or highly effective therapies based on a deep understanding of disease biology.
Pioneering work in gene-editing technologies through partnerships.
Indirect Competitors
- →
Gilead Sciences (and Kite Pharma)
Description:A major biopharmaceutical company with a growing oncology portfolio, especially in cell therapy through its subsidiary Kite Pharma. While not directly competing with Incyte's small molecules, their cell therapies (like CAR-T) treat some of the same cancers (e.g., lymphomas).
Threat Level:Medium
Potential For Direct Competition:Low in the near term, but their expansion in oncology could create future overlaps in targeted patient populations.
- →
Sanofi / Regeneron
Description:Their blockbuster biologic drug, Dupixent (dupilumab), is a major competitor in the atopic dermatitis market. It has a different mechanism of action (targeting IL-4 and IL-13) but competes for the same patients as Opzelura.
Threat Level:High
Potential For Direct Competition:Already a significant indirect competitor in the dermatology space.
- →
Novartis
Description:A global pharmaceutical giant and Incyte's ex-US commercialization partner for Jakafi. While a partner, Novartis also has a vast oncology portfolio and could develop or acquire assets that compete with Incyte's pipeline in the future.
Threat Level:Medium
Potential For Direct Competition:High, given their deep expertise in hematology and oncology and intimate knowledge of the ruxolitinib market.
Competitive Advantage Analysis
Sustainable Advantages
- Advantage:
First-Mover Advantage with Jakafi in Myelofibrosis
Sustainability Assessment:Jakafi is the well-established standard of care, giving it strong brand equity and physician loyalty. This is sustainable until a clearly superior therapy emerges.
Competitor Replication Difficulty:Medium
- Advantage:
First FDA-Approved Treatment for Vitiligo (Opzelura)
Sustainability Assessment:Significant advantage in a market with high unmet need. Sustainable for the near-to-medium term, but competitors are actively developing therapies for vitiligo.
Competitor Replication Difficulty:Medium
- Advantage:
Deep Expertise in JAK Inhibition
Sustainability Assessment:Extensive research and clinical development experience in the JAK pathway provides a scientific and intellectual property advantage that can be leveraged for future pipeline development.
Competitor Replication Difficulty:Hard
Temporary Advantages
{'advantage': 'Patent Protection on Key Products', 'estimated_duration': "Jakafi's primary patent is expected to face challenges and potential generic competition in the coming years, creating a significant revenue risk. "}
Disadvantages
- Disadvantage:
High Revenue Concentration on Jakafi
Impact:Critical
Addressability:Moderately
- Disadvantage:
JAK Class-wide Safety Concerns
Impact:Major
Addressability:Difficult
- Disadvantage:
Increased Competition in Core Markets
Impact:Major
Addressability:Difficult
Strategic Recommendations
Quick Wins
- Recommendation:
Amplify Digital Marketing for Opzelura
Expected Impact:High
Implementation Difficulty:Easy
- Recommendation:
Highlight Differentiated Safety/Efficacy Profile vs. Competitors
Expected Impact:Medium
Implementation Difficulty:Moderate
Medium Term Strategies
- Recommendation:
Accelerate Pipeline Diversification
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Expand Opzelura Indications
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Strategic Business Development and Acquisitions
Expected Impact:High
Implementation Difficulty:Difficult
Long Term Strategies
- Recommendation:
Invest in Novel Therapeutic Platforms Beyond Small Molecules
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Establish Leadership in Underserved Dermatological Conditions
Expected Impact:Medium
Implementation Difficulty:Difficult
Position Incyte as the 'Pioneering Leader in Targeted Therapies for Hematology and Dermatology.' This leverages the company's first-mover status with Jakafi and Opzelura while emphasizing the scientific precision of its portfolio. The 'Solve On' motto is a strong foundation for this narrative.
Differentiate through 'Science-Driven Patient Solutions.' Focus on being the best-in-class or first-in-class solution for specific, well-defined patient populations with high unmet needs. This contrasts with broader approaches from larger pharma, emphasizing depth of expertise over breadth of portfolio.
Whitespace Opportunities
- Opportunity:
Develop Therapies for Rare Inflammatory Skin Diseases
Competitive Gap:Many rare dermatological conditions lack effective approved treatments. Large pharma competitors often focus on larger markets like psoriasis or atopic dermatitis.
Feasibility:Medium
Potential Impact:High
- Opportunity:
Combination Therapies in Myelofibrosis
Competitive Gap:While competitors are challenging Jakafi as a monotherapy, there is an opportunity to establish Jakafi as the backbone of future combination regimens, thereby extending its lifecycle and market leadership.
Feasibility:High
Potential Impact:High
- Opportunity:
Patient-Centric Digital Health Solutions
Competitive Gap:Many competitors focus solely on the drug. Providing digital tools for symptom tracking, patient education, and community building for conditions like vitiligo or myeloproliferative neoplasms (MPNs) could build strong patient loyalty.
Feasibility:Medium
Potential Impact:Medium
Incyte operates in the mature and highly competitive biopharmaceutical industry, where innovation is paramount. The company has successfully carved out a strong position as a leader in targeted small molecule therapies, particularly through its expertise in the JAK inhibitor class. Its primary competitive advantage stems from the first-mover status of its flagship product, Jakafi, in myeloproliferative neoplasms (MPNs), and its groundbreaking approval of Opzelura for vitiligo, a market with significant unmet need.
The competitive landscape is intensifying. In hematology, Incyte faces direct challenges from companies like Bristol Myers Squibb, whose drug Inrebic targets the same indication as Jakafi. The larger threat, however, comes from the upcoming patent expiration of Jakafi, which accounts for the majority of Incyte's revenue and creates a critical need for diversification.
In the burgeoning dermatology space, Opzelura is well-positioned but faces formidable competition from global pharmaceutical giants AbbVie and Pfizer. Both companies are aggressively marketing their own JAK inhibitors (Rinvoq and Cibinqo, respectively) for atopic dermatitis, leveraging their immense commercial power and broad portfolios. Furthermore, Incyte faces indirect competition from biologics like Sanofi's Dupixent, which holds a strong position in the atopic dermatitis market.
The key strategic challenge for Incyte is to successfully navigate the transition from a company heavily reliant on a single blockbuster to a more diversified biopharmaceutical leader. This requires flawless execution in several areas: maximizing the lifecycle of the ruxolitinib franchise (both Jakafi and Opzelura), accelerating the development of its late-stage pipeline assets, and pursuing strategic acquisitions to bring in external innovation.
Opportunities exist in leveraging its deep scientific expertise to address other underserved specialty conditions in dermatology and inflammation. By focusing on being first-in-class or best-in-class in niche indications, Incyte can avoid direct, resource-intensive competition with larger players. The company's future success will depend on its ability to execute this diversification strategy effectively before the competitive pressures on Jakafi erode its primary revenue stream.
Messaging
Message Architecture
Key Messages
- Message:
Solve On.
Prominence:Primary
Clarity Score:Medium
Location:Homepage hero banner; recurring tagline
- Message:
Every challenge is a chance for a breakthrough.
Prominence:Secondary
Clarity Score:High
Location:Homepage hero banner, supporting 'Solve On.'
- Message:
Finding innovative solutions for serious unmet medical needs.
Prominence:Secondary
Clarity Score:High
Location:Mission statement, 'What We Do' section
- Message:
Scientific excellence driven by the need for innovative solutions for patients.
Prominence:Secondary
Clarity Score:High
Location:Homepage, 'What We Do' section link
- Message:
Passionate individuals bring our ideas to life and help to solve the toughest challenges.
Prominence:Tertiary
Clarity Score:High
Location:Homepage, 'Culture & Careers' section link
The messaging hierarchy is clearly established, with 'Solve On.' as the central, memorable brand mantra. This is effectively supported by secondary messages that explain what 'Solve On' means: persevering through challenges to find scientific solutions for patients. Tertiary messages about culture and careers are appropriately subordinated. The hierarchy successfully establishes a core brand idea and then provides layers of explanation.
The core message of 'Solve On.' is exceptionally consistent across the entire website, appearing in headlines, section titles ('Solve On. Stories'), and thematic content. Supporting messages about scientific innovation and patient focus are also repeated consistently, creating a unified and coherent narrative.
Brand Voice
Voice Attributes
- Attribute:
Determined & Resilient
Strength:Strong
Examples
- •
Solve On.
- •
Every challenge is a chance for a breakthrough.
- •
relentless pursuit to find answers for patients
- Attribute:
Scientific & Expert
Strength:Strong
Examples
- •
Scientific excellence
- •
discovery, development and commercialization of novel medicines
- •
deep knowledge and understanding of cellular oncogenic pathways
- Attribute:
Compassionate & Patient-Centric
Strength:Moderate
Examples
- •
solutions for patients
- •
Patients Inspire Our Science
- •
A source for information and support.
- Attribute:
Corporate & Professional
Strength:Strong
Examples
- •
INVESTOR INFORMATION
- •
Compliance & Transparency
- •
Governance
Tone Analysis
Inspirational and Purpose-Driven
Secondary Tones
- •
Authoritative
- •
Hopeful
- •
Formal
Tone Shifts
Shifts to a more formal, financial, and data-driven tone in the 'Investors' section.
Adopts a more personal and empathetic tone in the 'Patient Resources' and 'Our Stories' sections, particularly in patient experience stories.
Voice Consistency Rating
Excellent
Consistency Issues
No itemsValue Proposition Assessment
Incyte leverages relentless scientific innovation to discover and develop first-in-class medicines for serious, unmet medical needs, particularly in oncology and dermatology.
Value Proposition Components
- Component:
Scientific Discovery & Innovation
Clarity:Clear
Uniqueness:Somewhat Unique
- Component:
Focus on Unmet Medical Needs
Clarity:Clear
Uniqueness:Common
- Component:
Expertise in Oncology & Inflammation/Autoimmunity
Clarity:Clear
Uniqueness:Somewhat Unique
- Component:
Patient-Centered Mission
Clarity:Clear
Uniqueness:Common
Incyte's primary differentiator is not what it does (which is common in biopharma) but how it does it, encapsulated by the 'Solve On.' ethos. This frames the company as resilient, persistent, and challenge-oriented. While many competitors also focus on 'unmet needs' and 'innovation', the 'Solve On.' narrative provides a unique, memorable, and ownable brand character. The focus on being 'first-in-class' is a tangible differentiator that could be emphasized more clearly.
The messaging positions Incyte as a deeply scientific, resilient, and purpose-driven organization, distinct from larger, more diversified pharmaceutical giants like AbbVie or Novartis. It carves out a niche as a focused innovator. The messaging is less about market dominance and more about scientific leadership and solving difficult problems others may not be tackling.
Audience Messaging
Target Personas
- Persona:
Healthcare Professionals (HCPs)
Tailored Messages
- •
HCP Resources
- •
Portfolio (Oncology, Dermatology)
- •
Scientific Advancement (Stories)
Effectiveness:Effective
- Persona:
Patients & Caregivers
Tailored Messages
- •
Patient Resources
- •
Patient Experience (Stories)
- •
Disease Education (Stories)
Effectiveness:Effective
- Persona:
Investors & Partners
Tailored Messages
- •
Investor Information
- •
Press Releases
- •
Partnerships
Effectiveness:Effective
- Persona:
Potential Employees
Tailored Messages
- •
Culture & Careers
- •
Our Awesome Team
- •
Employee Culture (Stories)
Effectiveness:Effective
Audience Pain Points Addressed
- •
Lack of effective treatments for specific cancers and dermatological conditions ('serious unmet medical needs').
- •
The emotional and physical burden of living with diseases like vitiligo ('Reflections on Living with Vitiligo').
- •
Difficulty for HCPs in finding information on novel treatments.
Audience Aspirations Addressed
- •
Hope for a breakthrough treatment.
- •
Desire to contribute to meaningful scientific work (for employees).
- •
Opportunity for impactful financial returns through investing in life-changing science (for investors).
Persuasion Elements
Emotional Appeals
- Appeal Type:
Hope & Inspiration
Effectiveness:High
Examples
- •
'Every challenge is a chance for a breakthrough.'
- •
Patient stories detailing their journeys with a disease.
- •
The overarching 'Solve On.' theme, which suggests progress and ultimate success.
- Appeal Type:
Pride & Purpose
Effectiveness:Medium
Examples
- •
'Our Awesome Team'
- •
Stories under 'Employee Culture' and 'Community Impact'.
- •
Aimed at attracting talent and building employee morale.
Social Proof Elements
- Proof Type:
Media & News Coverage
Impact:Strong
Examples
A dedicated 'Latest News' and 'Press Releases' section.
- Proof Type:
Partnerships
Impact:Moderate
Examples
A 'Partnerships' link in the main navigation, indicating collaboration with other entities.
Trust Indicators
- •
Dedicated sections for 'Investors', 'Governance', and 'Compliance & Transparency'.
- •
Detailed information on their portfolio and drug development process.
- •
Clear contact information for Medical Information and locations.
- •
Use of scientific language and focus on data-driven progress.
Scarcity Urgency Tactics
No itemsCalls To Action
Primary Ctas
- Text:
Our Company
Location:Homepage Hero
Clarity:Clear
- Text:
View Story
Location:Our Stories Page
Clarity:Clear
- Text:
Our Patient Support
Location:Homepage
Clarity:Clear
- Text:
Open Positions
Location:Culture & Careers Dropdown
Clarity:Clear
The CTAs are primarily navigational, designed to guide different audiences to their respective sections of the site. They are clear, concise, and effective for this purpose. The site is not designed for direct conversion but for information dissemination and brand building. The CTAs successfully facilitate user journeys based on their likely intent (e.g., an investor easily finds the 'Investor Information' CTA).
Messaging Gaps Analysis
Critical Gaps
The connection between the high-level 'Solve On' brand promise and the specific, tangible benefits of their commercialized products (e.g., Jakafi, Opzelura) is not explicitly made on the corporate homepage. This is likely due to regulatory constraints but leaves a value gap for patient and HCP audiences.
Contradiction Points
No itemsUnderdeveloped Areas
While the 'Our Stories' section is strong, the narrative could be more directly integrated into the homepage to immediately showcase the human impact of the 'Solve On.' mission, rather than being a separate destination.
The 'Partnerships' messaging is underdeveloped. It's listed as a section but lacks a compelling narrative about why partners should choose Incyte and the mutual value of collaboration.
Messaging Quality
Strengths
- •
The 'Solve On.' tagline is a powerful, memorable, and consistent brand anchor.
- •
Excellent message discipline and consistency across the entire site.
- •
Clear segmentation and navigation for diverse audiences (Patients, HCPs, Investors, Careers).
- •
Effective use of storytelling to humanize the brand and its scientific mission.
Weaknesses
The core value proposition relies heavily on the ethos ('Solve On.') rather than on explicitly stated, unique capabilities or outcomes, which may be less compelling for audiences seeking hard data.
The messaging can feel abstract without the context provided by the deeper story pages.
Opportunities
- •
Quantify the impact of 'Solving On.' Where possible, use data or statistics to demonstrate the results of their persistence (e.g., 'Our persistence led to X number of patients treated').
- •
Create a more prominent 'innovation hub' that translates complex science (e.g., JAK inhibition) into more accessible language to showcase thought leadership.
- •
Develop more video content for the homepage to bring the 'Solve On.' stories to life more dynamically.
Optimization Roadmap
Priority Improvements
- Area:
Value Proposition
Recommendation:Sharpen the value proposition to more clearly articulate how Incyte's approach to science is different. Move beyond the 'what' (innovation for unmet needs) to the 'how' (e.g., 'Our integrated biology and chemistry teams enable us to tackle pathways others can't').
Expected Impact:High
- Area:
Homepage Narrative
Recommendation:Integrate 1-2 powerful, concise patient or scientist story snippets directly on the homepage. This would immediately connect the 'Solve On.' mantra to a real human outcome, making it more emotionally resonant.
Expected Impact:High
- Area:
Partnership Messaging
Recommendation:Build out the 'Partnerships' section with a clear value proposition for potential collaborators, highlighting success stories and outlining the benefits of working with Incyte's unique scientific culture.
Expected Impact:Medium
Quick Wins
On the homepage, change the sub-headline under 'WHAT WE DO' from a statement ('Scientific excellence driven by...') to a more active, benefit-oriented message for the audience (e.g., 'See how our scientific excellence delivers new solutions for patients').
Add a 'Featured Story' module to the homepage that rotates content from the 'Our Stories' section.
Long Term Recommendations
Develop a content strategy around the theme of 'Solving.' Create thought leadership content (articles, videos) that breaks down specific, complex scientific challenges the company has overcome, reinforcing the 'Solve On.' narrative with concrete proof.
Invest in creating a more interactive and visually engaging 'Portfolio' or 'Pipeline' section that explains the science and patient impact in a more accessible format than a simple list.
Incyte's strategic messaging is exceptionally disciplined, coherent, and effective, anchored by the powerful and memorable brand mantra, 'Solve On.' This central theme successfully frames the company's identity around resilience, scientific perseverance, and a patient-driven purpose. The message architecture is logical, with a clear hierarchy that reinforces this core idea across all sections of the site. The brand voice is consistent, blending scientific authority with an inspirational and determined tone, which is highly appropriate for the biopharmaceutical industry.
The website excels at audience segmentation, providing clear, distinct pathways for its key stakeholders—Patients, HCPs, Investors, and potential Employees. Each section is tailored with relevant information and calls-to-action that guide the user journey effectively. Persuasion is primarily achieved through emotional appeals to hope and purpose, backed by strong trust indicators like transparency in governance and a focus on scientific data.
The primary weakness is a slight over-reliance on the abstract 'Solve On.' concept at the highest level. While powerful, the messaging could be strengthened by more explicitly connecting this ethos to tangible outcomes and differentiated scientific capabilities on the homepage. The link between the brand promise and the specific value of their products is present but requires the user to navigate deeper into the site. The 'Our Stories' section is a major asset but its content could be leveraged more prominently to provide immediate, human-centered proof of the brand mission.
Opportunities for optimization lie in sharpening the uniqueness of their value proposition beyond industry-standard claims and creating a more dynamic, integrated narrative on the homepage. By bringing patient and scientist stories to the forefront and quantifying the impact of their resilient approach, Incyte can elevate its already strong messaging to create a more compelling and differentiated market position.
Growth Readiness
Growth Foundation
Product Market Fit
Strong
Evidence
- •
Blockbuster drug Jakafi® (ruxolitinib) with full-year 2024 revenues of $2.8 billion, showing consistent 8% YoY growth.
- •
Rapidly growing dermatology drug Opzelura® (ruxolitinib cream) with 50% YoY revenue growth in 2024, reaching $508 million.
- •
Successful launch of Niktimvo™ (axatilimab-csfr) for chronic graft-versus-host disease (GVHD), generating $14 million in its first two months.
- •
Broad global presence with operations in North America, Europe, and Japan, indicating international regulatory approval and market acceptance.
- •
Robust R&D pipeline with multiple late-stage assets and plans for at least three new Phase 3 initiations in 2025.
Improvement Areas
- •
Accelerate indication expansion for key products like Opzelura (e.g., pediatric atopic dermatitis, hidradenitis suppurativa) to maximize revenue potential before patent cliffs.
- •
Mitigate concentration risk associated with Jakafi by successfully commercializing new pipeline assets.
- •
Strengthen real-world evidence (RWE) generation to support value propositions for payers and health technology assessment (HTA) bodies.
Market Dynamics
Biopharmaceutical market estimated to grow at a CAGR of 8-14% from 2025, with the oncology segment growing at ~11%.
Mature
Market Trends
- Trend:
Growth in targeted therapies and precision medicine.
Business Impact:Strongly aligns with Incyte's portfolio of targeted JAK inhibitors (Jakafi, Opzelura) and its R&D focus. This trend validates their core scientific strategy and provides a favorable market environment for pipeline candidates.
- Trend:
Increasing dominance of biologics and novel modalities (ADCs, cell/gene therapies).
Business Impact:While strong in small molecules, Incyte needs to continue diversifying its modality capabilities to compete long-term. Strategic partnerships (e.g., with Syndax, Genesis Therapeutics) are a key vehicle for this.
- Trend:
Heightened payer scrutiny and market access challenges.
Business Impact:Increased pressure on pricing and reimbursement requires robust health economics and outcomes research (HEOR) to demonstrate value. This is a critical hurdle for all new product launches.
- Trend:
Shift in M&A towards bolt-on acquisitions and late-stage assets.
Business Impact:Creates opportunities for Incyte to acquire or in-license promising late-stage assets to de-risk its pipeline and fill revenue gaps.
Excellent. Incyte is well-positioned in high-growth therapeutic areas (oncology, dermatology) with a proven commercial engine and a promising late-stage pipeline, coinciding with major market demand for innovative, targeted treatments.
Business Model Scalability
High
Characterized by very high, fixed upfront R&D costs and clinical trial expenses, followed by relatively lower, scalable variable costs of manufacturing post-approval. This creates significant operating leverage once a drug is commercialized successfully.
High. Once a drug's revenue surpasses the initial R&D investment and ongoing marketing/manufacturing costs, a large portion of each additional dollar of sales contributes to profit.
Scalability Constraints
- •
Complex global supply chain and manufacturing scale-up for new products.
- •
Navigating disparate and lengthy regulatory approval processes in new geographic markets.
- •
Scaling specialized commercial and medical affairs teams to support new launches and indications.
Team Readiness
Strong. The executive team has a demonstrated track record of successfully navigating the full biopharma lifecycle from discovery through global commercialization (e.g., Jakafi, Opzelura).
Appears well-structured for a global biopharma company, with clear divisions for R&D, commercial, and corporate functions across key regions.
Key Capability Gaps
- •
Expertise in newer therapeutic modalities like cell and gene therapy may need to be acquired or further developed internally to stay at the cutting edge of innovation.
- •
Digital marketing and data analytics capabilities to optimize engagement with Healthcare Professionals (HCPs) in an evolving digital landscape.
- •
Market access and pricing negotiation expertise, especially for novel high-cost therapies in European and APAC markets.
Growth Engine
Acquisition Channels
- Channel:
Medical Science Liaisons (MSLs) & Key Opinion Leader (KOL) Engagement
Effectiveness:High
Optimization Potential:Medium
Recommendation:Leverage AI and data analytics to identify rising KOLs and predict scientific trends to engage thought leaders earlier in the drug development and commercialization lifecycle.
- Channel:
Direct Sales Force (to HCPs)
Effectiveness:High
Optimization Potential:Medium
Recommendation:Implement advanced CRM analytics to optimize call planning and personalize messaging based on HCP prescribing behavior and patient demographics. Enhance digital sales aids for more effective remote engagement.
- Channel:
Medical Conferences and Publications
Effectiveness:High
Optimization Potential:Low
Recommendation:Continue robust presence and data dissemination at major medical congresses. Focus on securing oral presentations for late-breaking pivotal trial data to maximize impact.
- Channel:
Patient Advocacy Group Engagement
Effectiveness:Medium
Optimization Potential:High
Recommendation:Deepen partnerships with patient advocacy groups to co-create educational resources and disease awareness campaigns, which can drive patient-led conversations with physicians, particularly in dermatology.
Customer Journey
The 'customer' journey involves both the prescribing HCP and the patient. It moves from awareness (clinical data, conferences) -> consideration (MSL visits, peer influence) -> prescription (HCP decision) -> fulfillment (pharmacy/specialty pharmacy) -> adherence (patient support programs).
Friction Points
- •
Reimbursement and prior authorization hurdles from payers.
- •
Lack of patient and/or HCP awareness of new indications for existing drugs.
- •
Managing patient adherence and potential side effects for chronic therapies.
Journey Enhancement Priorities
{'area': 'Payer & Reimbursement Support', 'recommendation': "Invest in dedicated 'hub' services that assist physician offices with benefits verification and prior authorization paperwork to reduce administrative burdens."}
{'area': 'Patient Onboarding & Adherence', 'recommendation': 'Expand digital patient support programs with personalized reminders, educational content, and telehealth nurse support to improve long-term adherence and outcomes for drugs like Opzelura. '}
Retention Mechanisms
- Mechanism:
Patient Support Programs ('IncyteCARES')
Effectiveness:High
Improvement Opportunity:Integrate digital health tools (e.g., mobile apps) to provide more dynamic support and gather real-world data on patient experience.
- Mechanism:
Ongoing Clinical Data Generation
Effectiveness:High
Improvement Opportunity:Systematically plan and execute long-term extension studies and real-world evidence studies to continuously reinforce the long-term safety and efficacy profile of key products, defending against new competitors.
- Mechanism:
Indication Expansion
Effectiveness:High
Improvement Opportunity:Strategically sequence new indication launches to create a continuous stream of 'new' news and reasons for HCPs to remain engaged with the brand and the company.
Revenue Economics
Strong. Biopharmaceutical products, particularly first-in-class or best-in-class oncology and dermatology drugs, typically have very high gross margins. The key challenge is recouping the massive upfront R&D investment.
Not directly applicable in the traditional sense. A proxy would be 'Lifecycle Revenue per Drug' vs. 'R&D and Commercialization Cost', which for successful drugs like Jakafi is extremely high.
High. The company reported a 15% increase in total revenues in 2024, demonstrating efficient commercial execution and strong market uptake of its key products.
Optimization Recommendations
- •
Optimize sales and marketing spend by using data analytics to focus resources on the highest-potential prescribers and geographic territories.
- •
Pursue strategic pricing and market access agreements that balance revenue maximization with broad patient access.
- •
Improve clinical trial efficiency through adaptive trial designs and use of AI to accelerate timelines and reduce costs.
Scale Barriers
Technical Limitations
- Limitation:
Clinical Trial Failures
Impact:High
Solution Approach:Diversify the R&D pipeline across multiple mechanisms of action and therapeutic areas. Employ rigorous 'Go/No-Go' criteria based on preclinical and early clinical data. Utilize advanced analytics and biomarkers to improve patient selection and increase probability of success.
- Limitation:
Chemistry, Manufacturing, and Controls (CMC) Scale-Up
Impact:Medium
Solution Approach:Invest in manufacturing capabilities and partnerships early in the development process. Utilize process analytical technology (PAT) to ensure quality and efficiency during scale-up.
Operational Bottlenecks
- Bottleneck:
Global Regulatory Approvals
Growth Impact:Delays global revenue generation and allows competitors to establish a foothold in key markets.
Resolution Strategy:Establish dedicated regional regulatory affairs teams with deep local expertise. Proactively engage with regulatory agencies (e.g., FDA, EMA, PMDA) throughout the clinical development process.
- Bottleneck:
Payer Reimbursement Negotiations
Growth Impact:Restricts patient access and limits commercial uptake even after regulatory approval.
Resolution Strategy:Integrate market access and HEOR teams into early-stage clinical development to ensure trial endpoints and data collection meet payer requirements.
Market Penetration Challenges
- Challenge:
Patent Expiration of Jakafi (approaching 2028)
Severity:Critical
Mitigation Strategy:Aggressively grow Opzelura revenues, accelerate the late-stage pipeline to launch new blockbusters, and actively pursue in-licensing or M&A of de-risked, revenue-generating assets.
- Challenge:
Intense Competition
Severity:Major
Mitigation Strategy:Compete on the basis of superior clinical data, demonstrating differentiated efficacy or safety. Build strong relationships with KOLs and prescribers. Continue to innovate through next-generation follow-on molecules.
- Challenge:
Crowded Markets (e.g., PD-1/PD-L1 inhibitors)
Severity:Major
Mitigation Strategy:Focus on niche indications, combination therapies, or patient populations with high unmet needs where Incyte's pipeline assets can demonstrate a clear advantage over established competitors.
Resource Limitations
Talent Gaps
- •
Computational Biologists and Data Scientists to leverage AI in drug discovery and clinical trial analysis.
- •
Experts in Cell and Gene Therapy to support diversification of the R&D portfolio.
- •
Global Market Access and HEOR specialists with experience in complex reimbursement environments.
Significant and ongoing capital is required to fund multiple large-scale Phase 3 trials, global product launches, and potential M&A or licensing deals.
Infrastructure Needs
Expansion of manufacturing capacity (either in-house or through CMOs) to support the growth of Opzelura and new product launches.
Investment in a unified data analytics platform to integrate clinical, commercial, and real-world data for strategic decision-making.
Growth Opportunities
Market Expansion
- Expansion Vector:
Indication Expansion for Approved Drugs
Potential Impact:High
Implementation Complexity:Medium
Recommended Approach:Prioritize and fully fund late-stage trials for Opzelura (hidradenitis suppurativa, prurigo nodularis) and povorcitinib to significantly expand the addressable patient populations for these key assets.
- Expansion Vector:
Geographic Expansion
Potential Impact:Medium
Implementation Complexity:High
Recommended Approach:Continue the methodical launch of Opzelura across Europe (Germany, France, Italy, Spain) and pursue approval in Japan and other key APAC markets.
Product Opportunities
- Opportunity:
Advance the Late-Stage Pipeline to Commercialization
Market Demand Evidence:Positive clinical data for assets like povorcitinib (dermatology), axatilimab (GVHD), and tafasitamab (oncology) address high unmet needs.
Strategic Fit:Excellent. Directly leverages Incyte's established expertise and commercial infrastructure in hematology/oncology and dermatology.
Development Recommendation:Execute flawlessly on pivotal Phase 3 trials and prepare pre-commercialization launch plans to ensure rapid uptake upon approval.
- Opportunity:
In-license or Acquire Mid-to-Late Stage Assets
Market Demand Evidence:The biopharma landscape is rich with smaller companies that have innovative assets but lack the capital or infrastructure for late-stage development and commercialization.
Strategic Fit:High. A key strategy to de-risk the pipeline and bridge the potential revenue gap from Jakafi's eventual loss of exclusivity.
Development Recommendation:Establish a dedicated, agile business development team with a clear mandate to identify and execute on bolt-on acquisitions in core therapeutic areas (oncology, dermatology, immunology).
Channel Diversification
- Channel:
Digital Opinion Leader (DOL) Engagement
Fit Assessment:High
Implementation Strategy:Develop a systematic program to identify and build relationships with influential HCPs on digital platforms (e.g., Twitter/X, Doximity) to shape clinical discourse and disseminate data.
- Channel:
Telehealth Provider Partnerships
Fit Assessment:Medium
Implementation Strategy:Explore pilot partnerships with major telehealth platforms, particularly in dermatology, to provide educational resources about conditions like atopic dermatitis and vitiligo and the potential role of Opzelura.
Strategic Partnerships
- Partnership Type:
Co-development / Co-commercialization
Potential Partners
Biotech companies with promising mid-stage assets (e.g., Syndax Pharma).
Larger pharma companies to share the risk/cost of mega-trials or to access complementary commercial infrastructure in specific geographies.
Expected Benefits:Shared R&D costs, access to external innovation, expanded commercial reach, and risk mitigation.
- Partnership Type:
AI / Tech Platform Collaborations
Potential Partners
AI-driven drug discovery companies (e.g., Genesis Therapeutics).
Real-world data and analytics firms.
Expected Benefits:Accelerate drug discovery, optimize clinical trial design, and generate compelling evidence for payers, improving R&D productivity.
Growth Strategy
North Star Metric
Total Annual Revenue from Non-Jakafi Products
This metric directly tracks the company's primary strategic imperative: diversifying its revenue base away from its reliance on Jakafi. It aligns the entire organization—from R&D to commercial—on the goal of successfully launching and scaling new products to ensure long-term, sustainable growth beyond the Jakafi patent cliff.
Achieve >25% YoY growth in this metric for the next three years.
Growth Model
Science-Led & Commercialization-Excellence Model
Key Drivers
- •
R&D Productivity (number of successful pivotal trials).
- •
Regulatory Success (approvals of new drugs and new indications).
- •
Commercial Launch Velocity (revenue trajectory in the first 24 months post-launch).
- •
Market Access (securing favorable reimbursement for new products).
Maintain a high R&D investment rate while building integrated, cross-functional launch readiness teams for each late-stage asset. These teams should include R&D, medical affairs, market access, and commercial leadership from at least 36 months prior to expected launch.
Prioritized Initiatives
- Initiative:
Maximize Opzelura Growth Engine
Expected Impact:High
Implementation Effort:Medium
Timeframe:Ongoing (next 12-24 months)
First Steps:Secure FDA approval for pediatric atopic dermatitis and aggressively prepare for a successful commercial launch. Finalize Phase 3 data readout for hidradenitis suppurativa.
- Initiative:
Execute Flawless Launch of Next Wave of Products
Expected Impact:High
Implementation Effort:High
Timeframe:12-36 months
First Steps:Finalize Phase 3 data for povorcitinib and tafasitamab; build out market access and commercial plans for these potential launches.
- Initiative:
Strategic Business Development / M&A
Expected Impact:High
Implementation Effort:Medium
Timeframe:Ongoing
First Steps:Define specific criteria for in-licensing/acquisition targets (therapeutic area, stage of development, financial model) and proactively engage with potential partners.
Experimentation Plan
High Leverage Tests
{'experiment_name': 'Phase 2 Proof-of-Concept Trials', 'hypothesis': 'Test novel pipeline candidates in well-defined patient populations to quickly establish efficacy and safety signals before committing to large, expensive Phase 3 programs.'}
{'experiment_name': 'Health Economics & Outcomes Research (HEOR) Studies', 'hypothesis': "Conduct studies that demonstrate a new drug's value beyond clinical efficacy (e.g., reduced hospitalizations, improved quality of life) to strengthen the reimbursement case with payers."}
Success is measured by clinical trial endpoints (for R&D) and market access/uptake metrics (for HEOR), ultimately leading to increased probability of regulatory approval and commercial success.
Aligned with the long-cycle nature of biopharma R&D; pipeline updates and clinical trial readouts occur on a semi-annual and annual basis.
Growth Team
Establish dedicated, empowered 'Asset Teams' for each late-stage and key commercial product. These cross-functional teams should be led by a General Manager and include representation from R&D, regulatory, commercial, and market access.
Key Roles
- •
Head of New Product Planning
- •
Vice President of Global Market Access
- •
Director of Business Development & Licensing
- •
Chief Data & Analytics Officer
Invest in training for digital marketing and omnichannel HCP engagement. Actively recruit talent from the tech and data science sectors to build internal analytics capabilities. Use strategic partnerships to gain experience in new therapeutic modalities.
Incyte is in a strong position for continued growth, but it is approaching a critical strategic inflection point. The company's growth foundation is solid, anchored by the highly successful Jakafi and the rapidly ascending Opzelura, demonstrating exceptional product-market fit in the high-growth oncology and dermatology sectors. The market timing is favorable, with demand for targeted therapies aligning perfectly with Incyte's core strengths.
The company's growth engine is proven and efficient, capable of discovering, developing, and commercializing blockbuster drugs. However, the primary scale barrier and strategic challenge is the heavy reliance on Jakafi, which faces a patent cliff around 2028. This creates a significant urgency to diversify revenue streams. The company's future growth will be almost entirely dependent on its ability to successfully convert its promising late-stage pipeline into a new portfolio of commercial products.
Key growth opportunities are clear: 1) Maximize the lifecycle of existing assets through aggressive indication and geographic expansion, particularly for Opzelura. 2) Ensure flawless execution on the late-stage pipeline, with assets like povorcitinib and tafasitamab representing the next wave of potential blockbusters. 3) Utilize its strong balance sheet for strategic, bolt-on M&A to in-license de-risked assets that can contribute to near-term revenue.
The recommended growth strategy is to shift the company's central focus, as measured by the North Star Metric of 'Total Annual Revenue from Non-Jakafi Products', from defending its current position to aggressively building its future. This requires a dual focus on internal execution—delivering on the pipeline—and external growth through strategic business development. Incyte has the scientific expertise, commercial infrastructure, and capital to succeed, but the next 36 months will be decisive in determining its ability to transition into its next phase of growth and solidify its position as a sustainable, multi-product biopharmaceutical leader.
Legal Compliance
Incyte maintains a comprehensive and easily accessible Privacy Policy, which is a significant strength. The policy details the types of personal information collected, the purposes for its use, and sharing practices. It specifically addresses requirements for different jurisdictions, including an annex for country-specific information, demonstrating a clear understanding of its global obligations under regulations like GDPR. The policy outlines user rights, such as access and deletion, and provides clear methods for users to exercise these rights via a dedicated web form, a toll-free number for California residents, and an email address ([email protected]). It also includes a dedicated 'Consumer Health Data Privacy Policy' which details the collection and use of sensitive health data, a critical component for a biopharmaceutical company. The policy explicitly states it does not knowingly collect data from children under 13, aligning with COPPA in the US.
The 'Terms of Use' are present and clearly accessible. They effectively limit Incyte's liability by stating that website information is provided 'AS-IS' and is for informational purposes only, not as a substitute for professional medical advice. The terms include standard but important clauses regarding intellectual property, disclaimers of liability for direct or indirect damages, and rules governing user-submitted information. They also disclaim responsibility for third-party websites linked from their site. These terms are robust and typical for a corporation in a highly regulated industry, serving to manage legal risk associated with the website's content.
Incyte has implemented a sophisticated cookie consent mechanism, likely using a solution from OneTrust, a leader in privacy management software. Upon visiting the site, a clear banner appears that does not use pre-ticked boxes for non-essential cookies and requires affirmative consent, which aligns with GDPR's opt-in requirements. The Cookie Policy is detailed and distinguishes between 'Strictly Necessary', 'Performance', 'Functional', and 'Targeting' cookies. Crucially, it provides a 'Cookie Settings' link allowing users to exercise granular control over each category. The policy also notes that targeting cookies are not used on patient-facing product or disease websites, showing a nuanced, risk-based approach to different types of content and audiences.
Incyte demonstrates a strong commitment to global data protection standards. The privacy policy is structured to address both GDPR and CCPA/CPRA. For GDPR, it mentions the rights of data subjects and acknowledges data transfers, stating they will take precautions to protect data transferred to countries with less stringent laws. For CCPA/CPRA, it provides a toll-free number for California residents and details rights specific to them, including how sensitive personal information is handled. The provision of a dedicated data subject request form centralizes and streamlines the process for users to exercise their rights, which is a best practice. The company also has a distinct 'Consumer Health Data Privacy Policy' addressing the collection of sensitive data like health conditions and biometric data, indicating compliance with newer state-level health privacy laws in the U.S.
The website shows a basic but important awareness of accessibility standards. The presence of a 'Skip to main content' link is a key feature for users relying on screen readers. A full audit would be required for a definitive assessment, but this initial feature suggests that accessibility has been considered. To fully align with Web Content Accessibility Guidelines (WCAG) and the Americans with Disabilities Act (ADA), a comprehensive review of heading structures, alt-text for all images, keyboard navigation, and color contrast ratios would be necessary. The current state is a good starting point but may not be fully compliant.
As a global biopharmaceutical company, Incyte is subject to strict regulations from bodies like the FDA in the US and the EMA in Europe regarding the promotion and communication of medical products. The website's structure, which separates resources for Healthcare Professionals (HCPs) from those for Patients, is a critical and well-executed compliance strategy. This segmentation helps prevent the off-label promotion or direct-to-consumer advertising of prescription drugs in jurisdictions where it is prohibited (like the EU). The 'Investor' section contains appropriate governance documents and is separate from medical information, aligning with SEC regulations for publicly traded companies. The 'Responsibility' section, with its link to 'Compliance & Transparency' and documents like the 'Declaration of Compliance for CA', further demonstrates a mature approach to industry-specific legal obligations, such as the Sunshine Act.
Compliance Gaps
- •
Lack of a visible, dedicated accessibility statement or policy detailing the level of WCAG conformance the website aims to achieve.
- •
While the Privacy Policy is comprehensive, the specific legal basis for processing data under GDPR (e.g., consent, legitimate interest, contract) for each type of data processing activity could be more explicitly stated for European users.
- •
The Terms of Use do not specify the governing law or jurisdiction for dispute resolution, which can create ambiguity for a global user base.
Compliance Strengths
- •
Robust and granular cookie consent mechanism powered by a leading compliance tool (OneTrust).
- •
Comprehensive, well-structured, and easily accessible Privacy Policy that addresses multiple global regulations (GDPR, CCPA/CPRA).
- •
Clear and distinct separation of website content for different audiences (Patients, HCPs, Investors), which is a key requirement for pharmaceutical marketing compliance.
- •
Provision of multiple, clear channels for users to exercise their data privacy rights, including a dedicated web form and a toll-free number.
- •
Dedicated 'Compliance & Transparency' section within their corporate responsibility framework, signaling a high level of commitment to legal and ethical standards.
Risk Assessment
- Risk Area:
Website Accessibility
Severity:Medium
Recommendation:Conduct a formal WCAG 2.1 AA accessibility audit to identify specific gaps. Publish an Accessibility Statement on the website to demonstrate commitment and provide information for users with disabilities. This mitigates the risk of ADA-related lawsuits, which are increasingly common.
- Risk Area:
Global Legal Ambiguity in Terms
Severity:Low
Recommendation:Update the 'Terms of Use' to include a 'Governing Law and Jurisdiction' clause. For a US-headquartered company, this would typically specify the laws of the State of Delaware and the jurisdiction of its courts. This provides legal certainty and manages risk in the event of a dispute.
- Risk Area:
GDPR Specificity
Severity:Low
Recommendation:Enhance the EU-specific annex of the Privacy Policy to explicitly map each data processing activity to its corresponding legal basis under Article 6 of the GDPR. While likely compliant, explicit mapping improves transparency and demonstrates meticulous adherence to the regulation for EU authorities.
High Priority Recommendations
Commission a full WCAG 2.1 AA accessibility audit and remediation plan to minimize legal risk under the ADA and improve usability for all individuals.
Add a 'Governing Law and Jurisdiction' clause to the Terms of Use to create legal certainty for the company and its global website users.
Overall, Incyte demonstrates a very strong and mature legal compliance posture, reflecting its status as a major global biopharmaceutical company. The company's legal positioning is a strategic asset, enabling market access across numerous highly regulated jurisdictions while building trust with patients, healthcare professionals, and investors. The use of sophisticated tools for cookie consent and well-drafted, comprehensive privacy and terms of use policies show a proactive and well-resourced approach to risk management. The clear segmentation of website content for different audiences is expertly handled and is fundamental to navigating the complex global restrictions on pharmaceutical marketing. The identified gaps in accessibility and minor ambiguities in legal documents are low-to-medium risk but represent opportunities for enhancement. By addressing these minor points, particularly by formalizing its commitment to web accessibility, Incyte can further solidify its position as a legally diligent and responsible industry leader, enhancing its brand reputation and minimizing potential legal challenges.
Visual
Design System
Corporate
Good
Developing
User Experience
Navigation
Horizontal Mega Menu
Clear
Good
Information Architecture
Logical
Clear
Light
Conversion Elements
- Element:
Our Company CTA Button (Homepage Hero)
Prominence:High
Effectiveness:Effective
Improvement:The button is visually clear, but could be tested with more action-oriented text like 'Discover Our Mission' to enhance user engagement.
- Element:
Section Navigation Links (Homepage)
Prominence:Medium
Effectiveness:Somewhat effective
Improvement:The red underline on hover is a subtle but potentially weak affordance. Consider a more pronounced style change, like a background color fill or bolder text, to improve click-through signaling.
- Element:
Filter/Search on 'Solve On. Stories' page
Prominence:Medium
Effectiveness:Effective
Improvement:The functionality is clear. Aesthetically, the input fields could be styled to better match the site's modern, clean design.
Assessment
Strengths
- Aspect:
Clean & Professional Aesthetic
Impact:High
Description:The website projects a credible and professional image, utilizing ample white space, high-quality imagery, and a consistent color palette. This is crucial for a biopharmaceutical company aiming to build trust with patients, healthcare professionals (HCPs), and investors.
- Aspect:
Clear Information Architecture
Impact:High
Description:The site is well-organized with a logical content hierarchy. Key audiences (Investors, Patients, HCPs, Job Seekers) can easily find relevant information through the intuitive main navigation and clearly delineated content blocks on the homepage.
- Aspect:
Compelling Brand Message: 'Solve On.'
Impact:High
Description:The core brand message 'Solve On.' is prominently and consistently featured, effectively communicating the company's commitment to innovation and perseverance in scientific research. This creates a strong, memorable brand identity.
Weaknesses
- Aspect:
Understated Interactive Feedback
Impact:Medium
Description:Hover states and interactive element feedback (like the red underlines on links) are very subtle. This can lead to a less engaging user experience and a weaker sense of interactivity, potentially causing users to miss clickable elements.
- Aspect:
Generic Content Layouts
Impact:Low
Description:While clean, the internal content pages (e.g., the 'Stories' page) use very conventional, templatized layouts. This can make content feel repetitive and less engaging, missing an opportunity for more dynamic visual storytelling.
- Aspect:
Lack of Direct Patient/HCP Conversion Paths on Homepage
Impact:Medium
Description:The homepage does an excellent job of corporate storytelling but lacks direct, prominent calls-to-action for its key audiences, such as 'Find Patient Support' or 'Access Resources for HCPs'. Users must navigate through menus to find these critical resources.
Priority Recommendations
- Recommendation:
Enhance Interactive Element Affordance
Effort Level:Low
Impact Potential:Medium
Rationale:Strengthening hover and active states for links and CTAs with bolder visual cues (e.g., color fills, background changes) will improve usability, increase user confidence and engagement, and likely boost click-through rates on key navigational elements.
- Recommendation:
Introduce Audience-Specific CTA Blocks on Homepage
Effort Level:Medium
Impact Potential:High
Rationale:Adding dedicated sections on the homepage with clear CTAs for Patients, HCPs, and Investors will significantly improve user flow. This reduces clicks and cognitive load, allowing key audiences to access critical information (like patient resources or clinical trial data) more efficiently.
- Recommendation:
Diversify Content Presentation
Effort Level:Medium
Impact Potential:Medium
Rationale:Incorporate more varied and visually engaging content modules, especially on pages like 'Stories'. Using elements like pull quotes, statistic call-outs, embedded videos, and varied image/text layouts will break up monotony and improve storytelling, keeping users more engaged with the content.
Mobile Responsiveness
Good
Based on the consistent and clean design, it's highly probable that the layout adapts well to different screen sizes. The use of card-based components and a clear grid structure typically translates effectively to mobile breakpoints.
Mobile Specific Issues
The main navigation likely collapses into a standard hamburger menu, which is functional but requires careful organization of menu items to remain intuitive.
Complex imagery in the hero section might need to be cropped or replaced with a mobile-optimized version to maintain visual impact and clarity on smaller screens.
Desktop Specific Issues
Large amounts of whitespace, while beneficial for a clean look, can sometimes make content feel sparse on very wide desktop monitors if not managed with max-width containers.
Overall Visual & UX Strategy Assessment
Incyte's website presents a strong, professional, and trustworthy digital presence, which is paramount in the biopharmaceutical industry. The overall design language is corporate, clean, and science-focused, effectively aligning with its brand identity as an innovator in oncology and autoimmune disease therapies. The central theme, 'Solve On.', is a powerful and memorable message that is well-integrated throughout the site, reinforcing the company's mission-driven culture.
1. Design System and Brand Identity
The site employs a developing but consistent design system. The color palette—primarily blues, white, and a vibrant magenta/red accent—is used effectively to create a professional and serious yet hopeful tone. Typography is clean and legible, with clear heading hierarchies. The brand logo and the 'Solve On.' tagline are consistently applied, reinforcing brand identity. However, the system lacks maturity in its interactive elements; hover and click states are too subtle, which slightly diminishes the user experience and could be a point of friction.
2. Visual Hierarchy and Information Architecture
The visual hierarchy on the homepage is effective. The hero section immediately establishes the company's purpose, followed by clearly delineated content blocks that guide users to key areas like 'What We Do', 'Culture & Careers', and 'Patient Resources'. This structure successfully caters to the primary audience segments: prospective employees, patients seeking information, and corporate stakeholders. The information architecture is logical and predictable, making it easy for users to find what they are looking for with minimal effort.
3. Navigation and User Flow
The primary navigation is a standard horizontal mega menu, which is a clear and intuitive pattern for a corporate site with this level of content complexity. It provides a good overview of the site's structure without overwhelming the user. The user flow from the homepage to secondary pages is straightforward. However, the flow could be optimized by incorporating more direct calls-to-action on the homepage tailored to specific user journeys (e.g., a prominent 'For Patients' button that leads directly to support resources).
4. Mobile Responsiveness and Cross-Device Experience
While this analysis is based on static images, the component-based design with clear grids and cards suggests a well-executed responsive design. The layout is uncluttered, which typically translates well to smaller viewports. Potential challenges on mobile would involve ensuring the mega-menu is organized logically within a hamburger menu and that the impactful hero imagery is adapted effectively for vertical screens.
5. Visual Conversion Elements and CTAs
The primary calls-to-action, such as the 'Our Company' button in the hero, are clear and well-placed. However, secondary CTAs and clickable link styling are a key area for improvement. The thin red underline on hover is a weak visual signifier. Adopting a more robust style, such as changing the background color or text weight, would provide better affordance and likely increase engagement with these crucial navigational links.
6. Visual Storytelling and Content Presentation
The website excels at high-level corporate storytelling, particularly through its powerful hero imagery that blends human elements with scientific concepts. The 'Solve On. Stories' page is a good initiative to humanize the brand. However, the presentation within this section is visually monotonous, relying on simple, repeated layouts. There is a significant opportunity to enhance storytelling by using more diverse and dynamic content modules, such as impactful statistics, blockquotes from researchers or patients, and a more varied grid system to create a more engaging and memorable narrative.
Discoverability
Market Visibility Assessment
Incyte has established a strong scientific and clinical brand authority, particularly as a pioneer in JAK inhibitors with its flagship product, Jakafi. Its digital presence reinforces this through dedicated sections for scientific portfolios, clinical trial information, and resources for healthcare professionals (HCPs). The company's narrative-driven content under 'Our Stories' aims to build an emotional connection and thought leadership around patient experience and scientific advancement, positioning them as more than just a drug manufacturer, but a patient-centric innovator.
Incyte's market share visibility is dominated by its key products, Jakafi (ruxolitinib) and Opzelura. Digitally, this translates to high search visibility for branded terms. However, they face intense competition from global pharmaceutical giants like Bristol-Myers Squibb, Novartis, GSK, and AbbVie in their core therapeutic areas. These larger competitors often have a greater digital 'share of voice' for non-branded, disease-centric keywords (e.g., 'atopic dermatitis treatment', 'myelofibrosis therapy'), representing both a threat and an opportunity for Incyte to capture more top-of-funnel traffic.
In the biopharma context, 'customer acquisition' refers to influencing prescribing habits of HCPs and educating patients. Incyte's website is well-structured to serve these distinct audiences with dedicated 'HCP Resources' and 'Patient Resources' sections. The potential is strong, as digital channels are crucial for educating these groups. The 'Disease Education' content hub is a key asset for attracting patients seeking information, which can empower them to discuss treatment options with their doctors. The primary opportunity is to enhance the depth and interactivity of resources for HCPs to make prescribing information more accessible and compelling.
The corporate website demonstrates a clear strategy for global reach, featuring links to numerous country-specific domains across Europe and Asia. This structure is essential for delivering geographically compliant and locally relevant information. The digital strategy should focus on ensuring these regional sites are not just corporate portals but are rich with localized content that addresses the specific needs and regulations of each market, thereby maximizing penetration and engagement with international HCPs and patient communities.
Incyte's digital content effectively covers its core therapeutic areas: Oncology, Hematology (specifically MPNs and GVHD), and Dermatology (IAI). The 'Our Stories' section provides broad coverage across patient experiences, scientific advancements, and disease education. However, there is an opportunity to deepen this coverage with more technical and data-rich content for specialists, such as summaries of clinical trial data, webinars with key opinion leaders, and detailed mechanism-of-action explainers to further demonstrate their scientific leadership.
Strategic Content Positioning
Incyte's content is strategically segmented for different audiences and journey stages. 'Disease Education' stories address the initial 'Awareness' phase. 'Patient Resources' and personal stories cater to the 'Consideration' and 'Support' phases. The 'HCP Resources' and detailed portfolio information are targeted at the 'Decision' phase for healthcare providers. The alignment is logical, but the journey could be made more seamless with clearer calls-to-action guiding users from disease awareness content to specific treatment information or support programs.
Incyte's 'Solve On' narrative and 'Our Stories' platform are excellent foundations for thought leadership. The key opportunity is to elevate their scientific leaders as prominent voices in the industry. This can be achieved by creating more content featuring their R&D team, publishing accessible summaries of their scientific publications, and hosting expert panels or webinars on emerging topics in oncology and dermatology. This would solidify their position as forward-thinking innovators beyond their specific products.
While Incyte excels at storytelling, competitors like AbbVie and Pfizer often provide more interactive digital tools for HCPs (e.g., dosing calculators, patient outcome simulators) and more extensive video content explaining complex disease mechanisms. A significant opportunity exists for Incyte to develop such data-driven and interactive content. Furthermore, creating comprehensive content hubs around specific conditions they treat, such as vitiligo or myelofibrosis, could create a 'one-stop resource' that outshines competitor offerings and captures significant organic search traffic.
The brand message 'Solve On' is consistently applied across the website, from the homepage banner to the overarching theme of 'Our Stories'. This narrative of perseverance and innovation is effectively woven into patient testimonials, employee features, and company mission statements. This consistency builds a strong, recognizable brand identity that differentiates Incyte as a focused and mission-driven organization in a field of giants.
Digital Market Strategy
Market Expansion Opportunities
- •
Develop comprehensive, unbranded content hubs for diseases addressed by pipeline candidates to build market awareness and establish authority before launch.
- •
Create localized thought leadership content for key international markets (e.g., EU, Japan) featuring regional medical experts to deepen geographic penetration.
- •
Expand into new content formats like podcasts or documentary-style video series to tell the 'Solve On' story in more engaging ways, reaching audiences on different platforms.
Customer Acquisition Optimization
- •
Create a dedicated, easily accessible portal for HCPs with clinical trial data, MOA videos, and on-demand webinars to streamline their information-gathering process.
- •
Launch targeted digital campaigns around key medical congresses, providing virtual access to presentations and data releases to engage HCPs who cannot attend in person.
- •
Develop interactive patient support tools, such as symptom trackers or appointment guides, to increase engagement and provide tangible value beyond information.
Brand Authority Initiatives
- •
Establish a 'Science & Innovation' section on the website, profiling key scientists and their research to personify Incyte's R&D prowess.
- •
Partner with influential medical associations and patient advocacy groups to co-create educational content, leveraging their credibility and reach.
- •
Translate complex clinical data into visually compelling formats like infographics and short videos for dissemination on social media and in digital PR.
Competitive Positioning Improvements
- •
Position Incyte as the definitive leader in JAK inhibitor science through content that explains the history, evolution, and future of this mechanism.
- •
Emphasize the 'first-in-class' and 'best-in-class' aspects of their portfolio through comparative content that highlights unique clinical benefits.
- •
Leverage their relatively smaller size to project an image of agility, focus, and a deeper connection to patient communities compared to larger, more diversified competitors.
Business Impact Assessment
Market share growth can be indirectly measured through digital metrics such as an increase in organic search visibility for key non-branded therapeutic terms, a higher 'share of voice' in online conversations versus competitors, and growth in direct traffic to product and HCP-specific web pages.
Success for HCPs can be measured by engagement rates with clinical content, downloads of scientific papers, and webinar registrations. For patients, key metrics include traffic to 'Patient Resources', engagement with support program information, and organic search ranking for patient-related disease queries.
Brand authority is reflected in the growth of branded search volume, backlinks from reputable medical and academic institutions, media mentions of Incyte's research, and social media engagement with posts about scientific innovation.
Benchmarking against competitors should involve tracking keyword rankings for strategic disease and treatment terms, analyzing the volume and sentiment of online conversations about Incyte's products versus rivals', and measuring audience engagement with their content compared to key competitors.
Strategic Recommendations
High Impact Initiatives
- Initiative:
Develop Unbranded Disease Education Hubs
Business Impact:High
Market Opportunity:Capture significant top-of-funnel search traffic from patients and HCPs researching conditions like vitiligo, atopic dermatitis, and myelofibrosis. Establishes Incyte as the primary educational authority, building trust before a brand is ever introduced.
Success Metrics
- •
Organic traffic to hub pages
- •
Top 3 rankings for key disease-related keywords
- •
User engagement rate (time on page, pages per session)
- •
Newsletter sign-ups from the hub
- Initiative:
Launch an 'HCP Clinical Excellence' Portal
Business Impact:High
Market Opportunity:Differentiate from competitors by providing a best-in-class digital resource for busy healthcare professionals. Making clinical data highly accessible and easy to digest can directly influence prescribing decisions and build loyalty among the medical community.
Success Metrics
- •
Number of HCP registrations/logins
- •
Downloads of clinical resources (papers, slide decks)
- •
Views of on-demand medical webinars
- •
Reduction in bounce rate on HCP pages
- Initiative:
Amplify Scientific Leadership via Multimedia Content
Business Impact:Medium
Market Opportunity:Strengthen Incyte's brand positioning as an innovator and scientific leader. By showcasing the experts behind the science, Incyte can build a stronger corporate reputation that attracts top talent, investors, and potential partners.
Success Metrics
- •
Video views and completion rates
- •
Social media shares and engagement on scientific content
- •
Media mentions of Incyte's scientists and research
- •
Traffic from social platforms to the main website
Incyte should pursue a focused 'Specialized Innovator' digital strategy. Instead of competing with Big Pharma on sheer volume, the strategy should concentrate on dominating the narrative in their specific areas of expertise (JAK inhibition, MPNs, vitiligo). The digital presence must consistently project deep scientific authority, unparalleled patient centricity through compelling stories, and superior resource accessibility for HCPs. This positions Incyte as the more focused, agile, and expert partner compared to its larger, more generalized competitors.
Competitive Advantage Opportunities
- •
Become the #1 online resource for JAK inhibitor information for both patients and clinicians.
- •
Build the most active and supportive online patient community resource for a key condition, such as vitiligo.
- •
Leverage the 'Solve On' narrative to build a powerful employer brand, attracting top scientific talent through stories of purpose-driven work and innovation.
Incyte has built a strong and professional digital presence centered on its corporate brand identity of 'Solve On.' The website effectively segments content for its diverse audiences—patients, healthcare professionals (HCPs), and investors. Its primary strength lies in its use of storytelling to humanize the brand and connect with stakeholders on an emotional level, particularly through its 'Our Stories' section which covers patient experiences, scientific progress, and community impact.
From a market visibility perspective, Incyte holds a solid position for its branded products, Jakafi and Opzelura. However, it faces a significant challenge from larger, better-funded competitors like Bristol-Myers Squibb, AbbVie, and Novartis, who often dominate the search landscape for broader, non-branded disease and treatment-related terms. This represents the single largest opportunity for digital growth: capturing users earlier in their information-seeking journey through comprehensive, unbranded educational content.
Strategically, the content is well-aligned with the typical patient and HCP journeys, offering awareness-level content, consideration-phase resources, and decision-making support. The messaging is consistent and reinforces the company's mission. The primary gap is in the depth of interactive and data-rich resources for HCPs. While competitors are developing sophisticated digital tools, Incyte's digital presence remains more informational and narrative-focused.
To elevate its competitive positioning, Incyte should double down on its identity as a 'Specialized Innovator.' The strategic imperative is to create digital 'centers of excellence' around its core diseases and its pioneering science in JAK inhibition. By building the definitive online resource hubs for conditions like vitiligo or myelofibrosis, and by creating an unparalleled clinical resource portal for HCPs, Incyte can build a defensible digital moat that is not dependent on advertising spend, but on genuine authority and value. This will not only drive organic growth and educate the market but also strengthen brand authority, attract top talent, and solidify its position as a leader in its chosen therapeutic fields.
Strategic Priorities
Strategic Priorities
- Title:
Project Horizon: Accelerate Late-Stage Pipeline to Commercialization
Business Rationale:The company faces a critical revenue concentration risk with its flagship drug, Jakafi, approaching its patent cliff around 2028. Aggressively advancing the late-stage pipeline is the most critical internal initiative to ensure new, high-margin revenue streams are established and scaled before this predictable revenue decline.
Strategic Impact:This initiative transforms Incyte's risk profile from a company dependent on a single blockbuster to a sustainable, multi-product biopharmaceutical leader. It directly addresses the primary long-term threat to the business, securing its future growth trajectory and investor confidence.
Success Metrics
- •
Revenue from products launched post-2023 > $1.5B by EOY 2027
- •
Achieve at least two new drug approvals (NDAs) from the current pipeline by Q4 2026
- •
Reduced revenue concentration: Non-Jakafi product revenue > 50% of total revenue by EOY 2028
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Revenue Model
- Title:
Execute Strategic Asset Acquisition Program to De-Risk Growth
Business Rationale:Relying solely on the internal pipeline is inherently risky and time-consuming. A disciplined M&A strategy to acquire or in-license de-risked, mid-to-late-stage assets in core therapeutic areas (oncology, dermatology) is the fastest way to bridge the potential revenue gap from Jakafi's patent expiration.
Strategic Impact:This program provides a crucial external lever for growth, de-risking the company's future by adding external innovation and near-term revenue potential. It allows Incyte to buy, rather than build, certain components of its future portfolio, ensuring a more predictable growth curve.
Success Metrics
- •
Deploy $2-4B in capital for strategic acquisitions/licensing within 24 months
- •
Acquire at least one Phase 3 or commercial-stage asset
- •
Projected revenue from acquired assets to exceed acquisition cost within 5 years
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Partnerships
- Title:
Establish Opzelura as a Standalone 'Mega-Blockbuster' Franchise
Business Rationale:Opzelura is the company's most significant current growth driver and the fastest organic path to revenue diversification. Creating a dedicated strategic focus to maximize its global market penetration and accelerate label expansion is paramount to reducing immediate reliance on Jakafi.
Strategic Impact:Elevating Opzelura to a multi-billion dollar, standalone franchise solidifies a second powerful revenue pillar for the company. This immediately strengthens the company's financial profile and demonstrates its ability to successfully commercialize major assets beyond its initial blockbuster.
Success Metrics
- •
Achieve >$2B in annual global Opzelura revenue by EOY 2027
- •
Secure regulatory approval for at least two new major indications (e.g., hidradenitis suppurativa)
- •
Capture >40% market share in key approved indications within 3 years of launch
Priority Level:HIGH
Timeline:Quick Win (0-3 months)
Category:Market Position
- Title:
Develop an Integrated Digital Ecosystem for Patient & Physician Engagement
Business Rationale:In crowded markets with large competitors, the product alone is not a sufficient differentiator. Building a best-in-class digital ecosystem with patient support tools and physician resources creates a competitive moat based on service and experience, fostering loyalty beyond the molecule.
Strategic Impact:This transforms the business from a drug provider to a comprehensive disease management partner. It creates strong brand preference, improves patient adherence, and generates valuable real-world data, creating a sustainable competitive advantage that is difficult for competitors to replicate.
Success Metrics
- •
Increase patient enrollment in digital support programs by 50% YoY
- •
Achieve >30% adoption of the HCP resource portal among target prescribers
- •
Demonstrate a measurable improvement in patient adherence rates for supported therapies
Priority Level:MEDIUM
Timeline:Strategic Initiative (3-12 months)
Category:Customer Strategy
- Title:
Diversify R&D into Next-Generation Therapeutic Platforms
Business Rationale:While Incyte excels at small molecule discovery, the future of biopharma lies in a diversity of therapeutic modalities (e.g., biologics, cell therapies, ADCs). To ensure long-term relevance and a competitive pipeline for the 2030s, the company must strategically invest in and build capabilities in these next-generation platforms.
Strategic Impact:This initiative future-proofs the company's innovation engine. It positions Incyte to tackle a broader range of diseases and biological targets, ensuring it remains at the cutting edge of science and can sustain its growth long after the current pipeline matures.
Success Metrics
- •
Establish at least two strategic partnerships or acquisitions in new modality areas within 18 months
- •
Allocate 15-20% of the R&D budget to non-small-molecule platforms by EOY 2026
- •
Advance a first-in-class candidate from a new platform into preclinical development
Priority Level:MEDIUM
Timeline:Long-term Vision (12+ months)
Category:Operations
Incyte must execute an urgent and aggressive pivot from its reliance on the Jakafi blockbuster to become a sustainable, multi-product biopharma leader. This requires a dual strategy of flawless internal execution—accelerating the late-stage pipeline and maximizing Opzelura—combined with external growth through strategic asset acquisitions to bridge the impending revenue gap.
The key competitive advantage Incyte must build is 'Specialized Leadership': being the undisputed scientific, clinical, and patient-support leader in niche, high-unmet-need therapeutic areas, creating a focused moat of expertise that larger, more diffuse competitors cannot easily replicate.
The primary growth catalyst is the successful commercialization of its 'next wave' pipeline. While Opzelura's growth is vital, the launch of two or more new blockbuster drugs before the Jakafi patent cliff is the singular driver that will unlock the next phase of the company's valuation and long-term growth.