eScore
modernatx.comThe eScore is a comprehensive evaluation of a business's online presence and effectiveness. It analyzes multiple factors including digital presence, brand communication, conversion optimization, and competitive advantage.
Moderna has high content authority and brand recognition, primarily linked to its COVID-19 vaccine, but is working to extend this to its broader pipeline. The website serves multiple audiences (investors, HCPs, talent) effectively. Its multi-channel presence is growing, with targeted digital campaigns on platforms like Doximity and social media, though it still trails larger competitors in overall digital visibility for emerging therapeutic areas.
Strong content authority and global brand recognition built during the pandemic, establishing a foundation of trust in its core mRNA science.
Systematically invest in organic search visibility for non-branded, disease-specific keywords (e.g., 'personalized oncology vaccines') to capture professional interest and reduce reliance on brand recognition.
Moderna's core message about the transformative power and speed of its mRNA platform is clear, consistent, and effectively differentiates it from traditional pharma. The company is actively working to shift its narrative from a 'COVID company' to a diversified 'mRNA platform leader', using campaigns like 'Welcome to the mRNAge'. While messaging for potential employees is strong, communication tailored to patients and investors is less developed.
Excellent at explaining complex science in a simple, accessible way ('So, what is mRNA?'), which builds foundational understanding and trust with a broad audience.
Develop a dedicated content stream for patient-centric stories to emotionally connect the science to its human impact, which is currently a significant gap in the messaging.
The website offers a low cognitive load with a clean layout and clear information architecture for its distinct audiences (investors, media, HCPs). However, its conversion goals—which are informational (e.g., read a report, view jobs)—are hindered by weak calls-to-action. Key CTAs are styled as simple text links, lacking the visual prominence needed to guide users effectively through critical journeys, thus creating friction and reducing engagement.
A logical, audience-centric information architecture that allows diverse user personas to find relevant information efficiently without feeling overwhelmed.
Redesign all key calls-to-action from text links to visually distinct buttons with a clear hierarchy (primary, secondary) to improve affordance and increase click-through rates on critical user paths.
Credibility is exceptionally high due to the successful global rollout of its COVID-19 vaccine, which serves as powerful customer success evidence. The company demonstrates strong third-party validation through partnerships with entities like Merck and government agencies. Transparency is robust, with detailed financial reporting, a comprehensive privacy policy addressing GDPR, and proper use of 'safe harbor' statements for investors, although the main Terms of Service document is outdated.
Demonstrated adherence to stringent industry-specific regulations from the SEC (Forward-Looking Statements) and FDA/EMA (controlled marketing language), mitigating major financial and legal risks.
Immediately conduct a comprehensive legal review and update of the 'Terms and Conditions of Use' to reflect the company's current global scale, commercial status, and risk profile since 2018.
Moderna's primary competitive advantage is its pioneering, digitally-integrated mRNA platform, which is highly sustainable and difficult for competitors to replicate, enabling unprecedented speed from design to clinic. This is further protected by a strong intellectual property portfolio. However, its first-mover advantage is being challenged as large, well-funded competitors like Pfizer/BioNTech and Sanofi are aggressively investing in and acquiring mRNA capabilities.
The programmable and rapid-response nature of the mRNA platform is a durable competitive advantage, positioning Moderna as uniquely capable of addressing future health crises and rapidly developing new treatments.
Vertically integrate key aspects of the supply chain for critical raw materials (e.g., lipids for LNPs) to create a cost and supply advantage, further strengthening its moat against competitors.
The platform-based business model is inherently scalable, allowing the development of multiple drug candidates simultaneously with high potential operational leverage once products are commercialized. The company has a diverse and extensive pipeline with 45 programs, market expansion signals via new manufacturing facilities in the UK and Canada, and a substantial cash reserve ($7.5B) to fund growth. The primary barrier is not technical scalability but the execution of complex global clinical trials and commercial launches for this broad portfolio.
An extensive and diverse clinical-stage pipeline across multiple high-value therapeutic areas (oncology, rare diseases) provides numerous paths to future growth and revenue diversification.
Aggressively hire senior commercial launch leaders and market access experts with track records in oncology and rare diseases to fill key capability gaps required for successful expansion.
The business model is coherent, leveraging a core technology platform to address critical unmet medical needs, which aligns well with target markets. However, the company is in a challenging transition, with a revenue model heavily dependent on a declining single product while facing massive R&D expenses to fund its future. The strategic focus is clear—pipeline execution—but the company's ability to achieve its goal of breaking even by 2028 is contingent on flawless clinical and commercial execution.
The business model directly addresses critical unmet needs in medicine (e.g., pandemic response, oncology) where the platform's speed and novel mechanism of action are highly valued and command premium pricing.
Systematically prioritize the R&D portfolio, potentially pausing or discontinuing lower-probability programs to focus capital and resources on late-stage assets with the highest chance of near-term commercial success.
Moderna established itself as a market co-leader in mRNA COVID-19 vaccines but is now an emerging, and much smaller, player in new therapeutic areas against giants like Pfizer, GSK, and Merck. Its pricing power was high during the pandemic but is now subject to significant competitive pressure in commercial markets like RSV. The company's primary market influence comes from its ability to innovate and set the pace for mRNA technology development, forcing larger competitors to react and invest.
Ability to influence market direction by pioneering new applications of mRNA technology, such as the personalized cancer vaccine, forcing the industry to follow its innovative lead.
Achieve best-in-class commercial execution for the new RSV vaccine (mRESVIA) to prove its ability to compete effectively against entrenched players in a traditional, non-pandemic market.
Business Overview
Business Classification
Biotechnology
Technology Platform
Pharmaceuticals & Biotechnology
Sub Verticals
- •
mRNA Therapeutics & Vaccines
- •
Infectious Diseases
- •
Immuno-Oncology
- •
Rare Diseases
- •
Autoimmune Diseases
Growth-to-Mature Transition
Maturity Indicators
- •
Successfully commercialized a global blockbuster product (Spikevax).
- •
Possesses a substantial cash reserve from pandemic-era sales.
- •
Maintains an extensive and diverse clinical-stage pipeline.
- •
Transitioning from single-product revenue dependency to a multi-product portfolio.
- •
Significant ongoing investment in R&D and manufacturing infrastructure.
Enterprise
Pivoting from Pandemic-Driven Hypergrowth to Pipeline-Driven Strategic Growth
Revenue Model
Primary Revenue Streams
- Stream Name:
Product Sales (Vaccines)
Description:Direct sales of its COVID-19 vaccine (Spikevax) and future approved products, primarily through large-scale contracts with national governments and healthcare distributors.
Estimated Importance:Primary
Customer Segment:Governments & Public Health Organizations
Estimated Margin:High
- Stream Name:
Grants and Collaboration Agreements
Description:Funding received from government agencies (e.g., BARDA, NIH) and strategic partners to co-develop new therapies and for pandemic preparedness initiatives.
Estimated Importance:Secondary
Customer Segment:Government Agencies & Corporate Partners
Estimated Margin:Variable
Recurring Revenue Components
Potential for seasonal revenue from updated COVID-19/Flu combination vaccines.
Future therapies for chronic or rare diseases requiring ongoing treatment.
Pricing Strategy
Value-Based Pricing & Government Contracting
Premium
Opaque
Pricing Psychology
Innovation Premium: Justifies higher prices based on novel technology and R&D investment.
Value-Based Pricing: Correlates price to the economic and health value delivered, such as reduced hospitalization costs.
Monetization Assessment
Strengths
- •
Demonstrated ability to rapidly scale manufacturing and commercialize a product globally.
- •
High-margin potential for first-in-class or best-in-class therapies derived from its platform.
- •
Strong government relationships established during the pandemic.
Weaknesses
Significant revenue concentration on a single product (Spikevax) with declining demand.
Future revenue is highly dependent on uncertain clinical trial outcomes and regulatory approvals.
Opportunities
- •
Successfully launch new products from the late-stage pipeline (e.g., RSV vaccine, combination vaccines).
- •
Secure advance purchase agreements for future pandemic threats.
- •
Monetize the mRNA platform through strategic licensing or co-development deals.
Threats
- •
Intense competition from other mRNA players (e.g., Pfizer/BioNTech) and traditional pharmaceutical companies.
- •
Pricing pressure from governments and healthcare payers.
- •
Patent litigation and intellectual property disputes.
- •
Shifts in public health priorities away from infectious diseases.
Market Positioning
Pioneering Scientific Innovator
Market Co-Leader (mRNA COVID-19 Vaccines); Emerging Player (Broader Biopharma)
Target Segments
- Segment Name:
Governments & Public Health Agencies
Description:National and international bodies responsible for public health, pandemic preparedness, and vaccine procurement.
Demographic Factors
- •
Country income level (high, middle, low)
- •
Population size
- •
Healthcare system structure
Psychographic Factors
- •
Risk aversion to public health crises
- •
Emphasis on national health security
- •
Desire for technologically advanced medical solutions
Behavioral Factors
- •
Bulk purchasing behavior
- •
Long-term contract negotiations
- •
Reliance on regulatory approvals and clinical data
Pain Points
- •
Slow response times to new health threats
- •
Inflexible supply chains for traditional medicines
- •
High cost of managing widespread disease
Fit Assessment:Excellent
Segment Potential:Medium
- Segment Name:
Healthcare Providers & Payers
Description:Hospitals, clinics, pharmacy chains, and insurance companies that administer treatments and manage reimbursement.
Demographic Factors
- •
Size of patient population
- •
Specialization (e.g., oncology, infectious disease)
- •
Geographic location
Psychographic Factors
Focus on clinical efficacy and patient outcomes
Sensitivity to cost-effectiveness and budget impact
Behavioral Factors
Adoption of new medical technologies
Adherence to treatment guidelines and formularies
Pain Points
- •
Lack of effective treatments for many diseases
- •
Administrative burden of new therapies
- •
High cost of innovative medicines
Fit Assessment:Good
Segment Potential:High
- Segment Name:
Patient Populations with Unmet Needs
Description:Individuals suffering from diseases with limited or no effective treatment options, such as specific cancers or rare genetic disorders.
Demographic Factors
Disease prevalence
Age groups affected
Psychographic Factors
High motivation to seek novel treatments
Active participation in patient advocacy groups
Behavioral Factors
Proactive search for clinical trials
Strong engagement with medical specialists
Pain Points
- •
Lack of hope or treatment options
- •
Severe impact of disease on quality of life
- •
Frustration with the pace of drug development
Fit Assessment:Good
Segment Potential:High
Market Differentiation
- Factor:
mRNA Platform Technology
Strength:Strong
Sustainability:Sustainable
- Factor:
Rapid Development & Manufacturing Speed
Strength:Strong
Sustainability:Sustainable
- Factor:
Brand Recognition & Scientific Credibility
Strength:Moderate
Sustainability:Sustainable
- Factor:
Integration of AI and Digital Technologies
Strength:Moderate
Sustainability:Temporary
Value Proposition
Delivering on the promise of mRNA science to create a new generation of transformative medicines for patients.
Excellent
Key Benefits
- Benefit:
Rapidly develop medicines and vaccines for emerging and existing diseases.
Importance:Critical
Differentiation:Unique
Proof Elements
Development of COVID-19 vaccine in record time.
Website states 'rapid design, research and testing... within days'.
- Benefit:
Address a wide spectrum of diseases currently untreatable with conventional medicine.
Importance:Critical
Differentiation:Somewhat unique
Proof Elements
Diverse pipeline spanning oncology, rare diseases, and infectious diseases.
Claims of 'limitless treatments' on the homepage.
- Benefit:
A platform approach that accelerates the development of multiple drug candidates simultaneously.
Importance:Important
Differentiation:Unique
Proof Elements
45 development programs and 36 ongoing clinical trials mentioned.
Emphasis on the platform's ability to create 'More medicines, faster'.
Unique Selling Points
- Usp:
Proprietary mRNA delivery systems and sequence optimization technology.
Sustainability:Long-term
Defensibility:Strong
- Usp:
Proven end-to-end capability from digital design to large-scale cGMP manufacturing.
Sustainability:Medium-term
Defensibility:Moderate
Customer Problems Solved
- Problem:
Lengthy and expensive traditional drug development timelines.
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Inability of traditional drug modalities to target certain disease-causing proteins.
Severity:Major
Solution Effectiveness:Partial
- Problem:
Lack of rapid-response capability for global pandemics.
Severity:Critical
Solution Effectiveness:Complete
Value Alignment Assessment
High
The business model directly addresses critical unmet needs in public health (pandemic response) and medicine (oncology, rare diseases) where speed and novel mechanisms of action are highly valued.
High
The value proposition resonates strongly with governments seeking health security, providers needing better treatments, and patients desperate for new options.
Strategic Assessment
Business Model Canvas
Key Partners
- •
Governments (BARDA, NIH)
- •
Regulatory Agencies (FDA, EMA)
- •
Academic Research Institutions
- •
Contract Manufacturing Organizations (CMOs)
- •
Pharmaceutical and Biotech collaborators (e.g., Merck for cancer vaccines)
Key Activities
- •
Research & Development
- •
Clinical Trial Management
- •
mRNA Manufacturing & Supply Chain Logistics
- •
Regulatory Affairs
- •
Commercialization & Market Access
Key Resources
- •
Intellectual Property Portfolio (Patents on mRNA technology)
- •
Scientific and Clinical Expertise
- •
Proprietary Digital Drug Design Platform
- •
Manufacturing Facilities
- •
Significant Cash Reserves
Cost Structure
- •
R&D Expenses (primary cost driver)
- •
Cost of Goods Sold (COGS)
- •
Selling, General & Administrative (SG&A) Expenses
- •
Capital Expenditures for Manufacturing Facilities
Swot Analysis
Strengths
- •
Clinically and commercially validated mRNA platform.
- •
Strong global brand recognition and reputation for innovation.
- •
Robust balance sheet with significant cash for R&D and strategic acquisitions.
- •
Extensive and diverse pipeline across multiple high-value therapeutic areas.
Weaknesses
- •
Current over-reliance on a single product line (COVID-19 vaccine) for revenue.
- •
High stock market valuation creates pressure for continued blockbuster successes.
- •
Lack of long-term data on mRNA technology compared to traditional biologics.
Opportunities
- •
Become the market leader in new vaccine categories (e.g., RSV, CMV, combination flu/COVID).
- •
Achieve breakthrough results in difficult-to-treat areas like oncology and rare diseases.
- •
Leverage AI and automation to further accelerate drug discovery and reduce costs.
- •
Expand global manufacturing footprint to improve supply chain resilience.
Threats
- •
Intensifying competition from well-funded biotech and large pharma companies.
- •
Patent infringement lawsuits challenging core IP.
- •
Stringent regulatory hurdles for novel therapies.
- •
Potential for unforeseen long-term side effects to emerge, impacting platform credibility.
Recommendations
Priority Improvements
- Area:
Pipeline Execution
Recommendation:Aggressively advance late-stage clinical candidates (RSV, oncology) towards regulatory submission and commercial launch to de-risk the revenue profile.
Expected Impact:High
- Area:
Capital Allocation
Recommendation:Utilize the substantial cash position for strategic acquisitions of complementary technologies (e.g., novel delivery systems) or late-stage assets to accelerate diversification.
Expected Impact:High
- Area:
Market Narrative
Recommendation:Proactively manage investor and market expectations, shifting the narrative from a 'COVID company' to a diversified, platform-based biopharmaceutical leader.
Expected Impact:Medium
Business Model Innovation
Explore a 'Platform-as-a-Service' model, licensing the mRNA platform and manufacturing capabilities to other biotech/pharma companies for non-core therapeutic areas to generate ancillary revenue.
Develop a personalized medicine business unit, leveraging the platform's flexibility for individualized cancer vaccines and therapies.
Revenue Diversification
- •
Prioritize development of combination vaccines (e.g., COVID/Flu/RSV) to create a valuable seasonal franchise.
- •
Establish a strong commercial footprint in oncology and rare diseases, which offer durable revenue streams with high unmet need.
- •
Secure further government contracts for pandemic preparedness, establishing a recurring revenue stream for 'warm-base' manufacturing readiness.
Moderna's business model represents a paradigm shift in pharmaceutical development, leveraging a versatile technology platform (mRNA) to dramatically accelerate the R&D-to-market timeline. Its hypergrowth, fueled by the unprecedented success of the Spikevax vaccine, has established it as a major player in the biotechnology industry. The company's core strength lies in its validated platform, substantial cash reserves, and an ambitious pipeline targeting high-value markets like oncology and rare diseases.
However, the model faces a critical inflection point. The primary challenge is evolving from a single-product success story, heavily reliant on a declining COVID-19 market, into a sustainable, multi-product biopharmaceutical enterprise. The business model's future viability is entirely dependent on its ability to successfully execute on its clinical pipeline and commercialize new therapies. The transition from government contracts to navigating complex commercial payer landscapes for various diseases will test the organization's strategic agility.
Strategic priorities must center on disciplined pipeline execution, astute capital allocation for M&A, and managing market expectations. The company is well-positioned, but its valuation and future growth are predicated on transforming the scientific promise of its broad pipeline into a diversified portfolio of commercial products. Success in this evolution will solidify its position as a durable leader in modern medicine; failure would risk it becoming a one-hit wonder of the pandemic era.
Competitors
Moderna, a pioneer in mRNA technology, finds itself at a critical strategic inflection point. Its meteoric rise, fueled by the unprecedented success of the Spikevax COVID-19 vaccine, has established it as a major player in the biotechnology industry. However, the competitive landscape is rapidly evolving. The primary challenge for Moderna is to transition from a company largely dependent on a single product line, facing declining demand, to a diversified biopharmaceutical powerhouse proving the broad applicability of its mRNA platform. Its main direct competitor, BioNTech (with Pfizer), presents a formidable challenge with a similar, highly effective mRNA vaccine and the backing of Pfizer's global commercial infrastructure. The competitive arena is expanding beyond COVID-19 into new battlegrounds like RSV, influenza, and personalized cancer vaccines, where Moderna faces both established pharmaceutical giants and other agile biotech firms. Indirect competition comes from companies with alternative vaccine technologies like Novavax and large pharmaceutical companies such as Merck, Roche, and Bristol Myers Squibb, which dominate therapeutic areas like oncology that Moderna is targeting. Moderna's core sustainable advantage is its deep expertise and integrated platform for rapid mRNA therapeutic design and manufacturing. However, this technological lead is being challenged as competitors, including large-cap pharma like Sanofi, aggressively invest in and acquire mRNA capabilities. Strategic success will be defined by Moderna's ability to execute on its diverse pipeline, achieve key regulatory approvals, and successfully commercialize new products in competitive markets, thereby demonstrating long-term value beyond the pandemic.
Competitive Landscape
Growth
Oligopoly
Barriers To Entry
- Barrier:
High R&D and Clinical Trial Costs
Impact:High
- Barrier:
Stringent Regulatory Approval Processes (e.g., FDA, EMA)
Impact:High
- Barrier:
Intellectual Property & Patent Protection
Impact:High
- Barrier:
Complex Manufacturing & Scaling Capabilities
Impact:High
- Barrier:
Access to Distribution and Commercialization Networks
Impact:Medium
Industry Trends
- Trend:
Expansion of mRNA technology beyond infectious diseases into oncology, rare diseases, and autoimmune disorders.
Impact On Business:Creates significant growth opportunities but also brings Moderna into direct competition with established pharmaceutical giants in these therapeutic areas.
Timeline:Immediate
- Trend:
Increased focus on personalized medicine, such as individualized cancer vaccines.
Impact On Business:Aligns perfectly with the customizability of the mRNA platform, offering a key area for differentiation. Moderna is actively pursuing this with partners like Merck.
Timeline:Near-term
- Trend:
AI and Machine Learning in drug discovery and development.
Impact On Business:Offers opportunities to accelerate pipeline development and increase efficiency, a key factor in maintaining a competitive edge. Moderna's internal adoption, as noted on its website, is a positive indicator.
Timeline:Immediate
- Trend:
Increased M&A activity by large pharma to acquire novel technologies like mRNA.
Impact On Business:Intensifies competition as well-funded players like Sanofi acquire mRNA expertise, but also validates the high value of Moderna's platform and could present partnership opportunities.
Timeline:Near-term
Direct Competitors
- →
BioNTech (in partnership with Pfizer)
Market Share Estimate:Comparable to Moderna in the COVID-19 mRNA vaccine market, with regional variations.
Target Audience Overlap:High
Competitive Positioning:A science-driven mRNA pioneer partnered with a global pharmaceutical giant for unparalleled manufacturing, distribution, and marketing power.
Pipeline Focus
- •
Infectious Diseases (COVID-19, Influenza)
- •
Oncology (Personalized Cancer Vaccines)
- •
Rare Diseases
Strengths
- •
Strategic partnership with Pfizer provides massive global commercialization and manufacturing infrastructure.
- •
Strong brand recognition and trust from their COVID-19 vaccine, Comirnaty.
- •
Solid financial position from vaccine sales to fund R&D.
- •
Focused and respected scientific leadership.
Weaknesses
- •
Heavy reliance on the Pfizer partnership for commercial success, potentially limiting strategic independence.
- •
Also faces the challenge of diversifying revenue beyond the COVID-19 vaccine.
- •
Pipeline is heavily focused on mRNA, sharing similar platform risks with Moderna.
Differentiators
The Pfizer partnership is their single biggest differentiator, providing scale and market access that is difficult to replicate.
Early focus was on oncology, giving them deep, long-standing expertise in that specific application of mRNA.
- →
CureVac
Market Share Estimate:Minimal
Target Audience Overlap:Medium
Competitive Positioning:A foundational mRNA company focused on a differentiated second-generation platform, aiming for improved stability and efficacy.
Pipeline Focus
- •
Prophylactic Vaccines (COVID-19, Influenza)
- •
Oncology
- •
Molecular Therapy
Strengths
- •
Strategic partnerships with established players like GSK and Sanofi.
- •
Pioneering history in mRNA technology provides a deep knowledge base.
- •
Focus on second-generation mRNA technology could yield advantages in the future.
Weaknesses
- •
Significant setback with their first-generation COVID-19 vaccine, which failed to meet efficacy endpoints, damaging credibility and market position.
- •
Trails significantly behind Moderna and BioNTech in clinical development and commercialization.
- •
Faces an uphill battle to regain investor and public confidence.
Differentiators
Proprietary RNAoptimizer® platform which aims to optimize mRNA molecules for specific therapeutic purposes.
- →
Sanofi
Market Share Estimate:Emerging
Target Audience Overlap:Medium
Competitive Positioning:A global pharmaceutical leader aggressively investing to become a major force in mRNA through strategic acquisitions and internal development.
Pipeline Focus
- •
Infectious Diseases (Influenza)
- •
Oncology
- •
Immunology
- •
Rare Diseases
Strengths
- •
Massive global R&D, manufacturing, and commercial infrastructure.
- •
Significant financial resources to fund ambitious projects and acquisitions.
- •
Acquired Translate Bio for $3.2 billion to fast-track mRNA capabilities.
- •
Diversified portfolio outside of mRNA reduces overall company risk.
Weaknesses
- •
Late entrant to the mRNA space compared to pioneers like Moderna and BioNTech.
- •
Integrating acquired companies and building a cohesive mRNA culture can be challenging for a large, established organization.
- •
Initial mRNA vaccine efforts have lagged behind the market leaders.
Differentiators
Strategy of combining deep traditional pharma expertise with acquired, cutting-edge mRNA technology.
Dedicated mRNA 'Center of Excellence' signifies strong corporate commitment.
Indirect Competitors
- →
Novavax
Description:Develops protein subunit-based vaccines, offering a more traditional technological alternative to mRNA for infectious diseases like COVID-19 and influenza.
Threat Level:Medium
Potential For Direct Competition:Low (as they use a fundamentally different technology platform)
- →
Merck & Co., Inc.
Description:A global pharmaceutical leader with a dominant position in oncology (e.g., Keytruda), a key growth area for Moderna's personalized cancer vaccine. Merck is also a partner with Moderna on this vaccine.
Threat Level:High
Potential For Direct Competition:High (They are simultaneously a partner in personalized cancer vaccines and a competitor with their existing oncology portfolio).
- →
Roche (Genentech)
Description:One of the world's largest biotech companies with a massive portfolio in oncology and a leader in personalized healthcare.
Threat Level:High
Potential For Direct Competition:High (in the oncology space)
- →
Bristol Myers Squibb
Description:A major player in oncology and immunology with blockbuster drugs that would compete with Moderna's future therapies in these areas.
Threat Level:High
Potential For Direct Competition:High (in oncology and immunology)
Competitive Advantage Analysis
Sustainable Advantages
- Advantage:
Pioneering mRNA Platform Technology
Sustainability Assessment:Highly sustainable. Moderna's deep, focused expertise and integrated platform for rapid design, development, and manufacturing of mRNA candidates is a core, defensible asset.
Competitor Replication Difficulty:Hard
- Advantage:
Extensive Intellectual Property Portfolio
Sustainability Assessment:Sustainable for the life of the patents, but subject to legal challenges which are common in the industry.
Competitor Replication Difficulty:Hard
- Advantage:
Agile and Innovative Corporate Culture
Sustainability Assessment:Moderately sustainable. Their ability to move quickly was proven during the pandemic, but maintaining this agility as the company scales will be a challenge.
Competitor Replication Difficulty:Medium
Temporary Advantages
{'advantage': 'Exceptional Brand Recognition from COVID-19', 'estimated_duration': '2-4 years. This advantage will diminish as the pandemic recedes and other products enter the market.'}
{'advantage': 'Strong Financial Position from Spikevax Sales', 'estimated_duration': 'N/A. The cash reserves are durable, but the revenue stream generating them is declining rapidly. This capital must be deployed effectively to build future value. '}
Disadvantages
- Disadvantage:
High Dependence on a Single Product (Spikevax)
Impact:Critical
Addressability:Moderately (Dependent on successful execution of the R&D pipeline)
- Disadvantage:
Declining Revenue and Profitability Post-Pandemic
Impact:Major
Addressability:Difficult (Market forces are largely outside of their control; reliant on launching new blockbuster products).
- Disadvantage:
Intense Competition in Key Pipeline Areas (e.g., RSV, Oncology)
Impact:Major
Addressability:Moderately (Requires superior clinical data and effective commercial execution against entrenched competitors).
Strategic Recommendations
Quick Wins
- Recommendation:
Launch a targeted digital campaign highlighting the breadth of the pipeline beyond COVID-19 to reshape public and investor perception.
Expected Impact:Medium
Implementation Difficulty:Easy
- Recommendation:
Amplify messaging around the use of AI in their platform to reinforce the image of a tech-forward, innovative company.
Expected Impact:Low
Implementation Difficulty:Easy
Medium Term Strategies
- Recommendation:
Prioritize and accelerate development of the personalized cancer vaccine (PCV) in partnership with Merck, as it represents a truly differentiated, high-value asset.
Expected Impact:High
Implementation Difficulty:Moderate
- Recommendation:
Secure a strategic partnership for a non-mRNA asset to begin diversifying platform risk and supplement the pipeline.
Expected Impact:Medium
Implementation Difficulty:Difficult
- Recommendation:
Achieve best-in-class commercial execution for the new RSV vaccine (mRESVIA) to prove commercial capabilities beyond a pandemic setting.
Expected Impact:High
Implementation Difficulty:Moderate
Long Term Strategies
- Recommendation:
Establish clear market leadership in one therapeutic area outside of infectious disease vaccines (e.g., rare metabolic diseases or oncology) to prove the platform's long-term, diversified value.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Vertically integrate key aspects of the supply chain for raw materials (e.g., lipids for LNPs) to create a cost and supply advantage.
Expected Impact:Medium
Implementation Difficulty:Difficult
Position Moderna as 'The Digital Biotechnology Company,' emphasizing the programmability of mRNA and the use of AI to rapidly design novel medicines, moving the narrative from a vaccine company to a broad therapeutic platform company.
Differentiate based on speed and personalization. Focus on being the fastest from sequence to clinic across multiple therapeutic areas and lead the charge in bespoke therapies like personalized cancer vaccines.
Whitespace Opportunities
- Opportunity:
Develop mRNA therapies for rare genetic diseases, particularly those involving protein deficiencies.
Competitive Gap:Many rare diseases have no effective treatments, and the ability of mRNA to instruct cells to produce correct proteins is a perfect fit. This is a less crowded space than oncology.
Feasibility:High
Potential Impact:High
- Opportunity:
mRNA for autoimmune diseases.
Competitive Gap:Current treatments are often broadly immunosuppressive. mRNA could be used to induce antigen-specific tolerance, a more targeted and potentially revolutionary approach.
Feasibility:Medium
Potential Impact:High
- Opportunity:
In-vivo gene editing.
Competitive Gap:Using mRNA to deliver gene-editing tools (like CRISPR-Cas9 components) directly into the body could be safer and more transient than viral delivery methods.
Feasibility:Low
Potential Impact:High
- Opportunity:
Regenerative medicine applications.
Competitive Gap:Exploring the use of mRNA to stimulate tissue repair and regeneration, for example in cardiovascular or neurodegenerative diseases, is a nascent field with few established players.
Feasibility:Low
Potential Impact:High
Messaging
Message Architecture
Key Messages
- Message:
mRNA science can create a new generation of transformative medicines for patients.
Prominence:Primary
Clarity Score:High
Location:Homepage, Mission Statement
- Message:
Our mRNA platform enables the rapid design and production of new medicines for many diseases.
Prominence:Secondary
Clarity Score:High
Location:Homepage 'So, what is mRNA?' section
- Message:
Moderna is a fast-paced, purpose-driven, and innovative place to work and build a career.
Prominence:Secondary
Clarity Score:High
Location:Homepage 'Join us' section, Careers pages, Employee blogs
- Message:
Moderna is expanding its pipeline into new therapeutic areas like oncology and rare diseases.
Prominence:Tertiary
Clarity Score:Medium
Location:Blog Posts, Press Releases
The message hierarchy on the homepage is somewhat fragmented. While the core mission and the science of mRNA are present, they compete for attention with corporate news (earnings reports), job opportunities, and impact reports. The primary message about the transformative power of mRNA could be more dominant and serve as a clearer anchor for the rest of the page content.
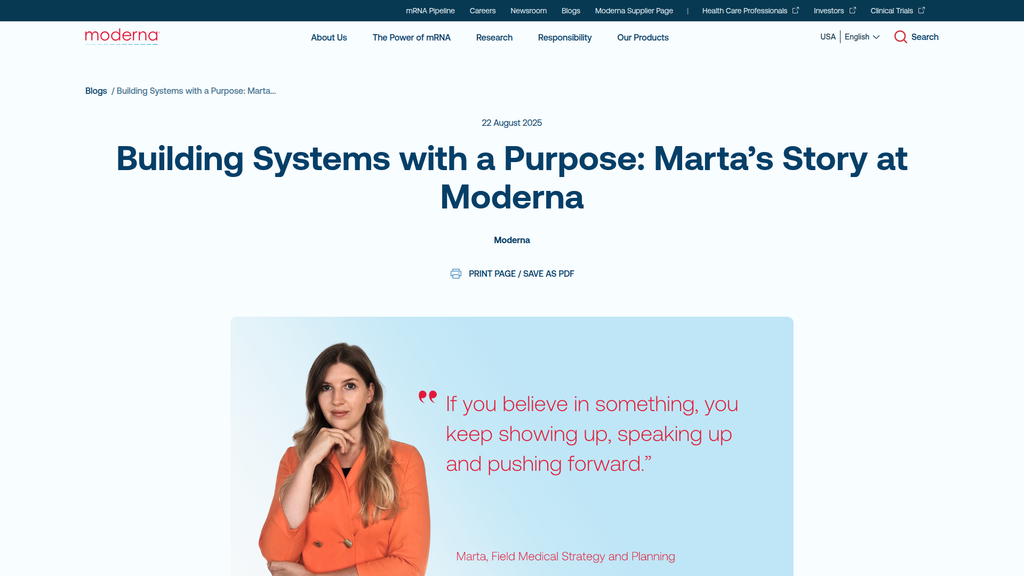
Messaging is highly consistent. The core ideas of speed, innovation, the power of the mRNA platform, and making an impact are woven through the corporate narrative, the scientific explanations, and the employee stories. The blog post about Marta effectively reinforces the company-level value proposition of being 'purpose-driven' and 'agile'.
Brand Voice
Voice Attributes
- Attribute:
Scientific & Authoritative
Strength:Strong
Examples
Messenger RNA (mRNA) already exists in your body.
mRNA medicines fight diseases in a different way than traditional medicine by prompting your immune system to create the tools to treat or prevent disease.
- Attribute:
Ambitious & Visionary
Strength:Strong
Examples
- •
Our mission is to deliver on the promise of mRNA science to create a new generation of transformative medicines for patients.
- •
We believe that if mRNA can treat one disease, it can treat many diseases.
- •
Join us and change the world of medicine
- Attribute:
Purpose-Driven & Human
Strength:Moderate
Examples
- •
It's about seeing each person as more than just a number.
- •
She was seeking meaningful impact.
- •
That kind of thoughtfulness, in a high-pressure environment, makes all the difference.
Tone Analysis
Forward-looking
Secondary Tones
- •
Educational
- •
Inspirational
- •
Corporate
Tone Shifts
Shifts from a high-level, visionary corporate tone on the homepage to a more personal, narrative tone in the employee blog posts.
Voice Consistency Rating
Good
Consistency Issues
The primary voice is consistent. However, the tone can feel slightly disjointed on the homepage, moving from a corporate/investor focus ('Earnings Report') to a scientific/public focus ('what is mRNA?') to a recruitment focus ('Join us') without a strong narrative thread connecting them.
Value Proposition Assessment
Moderna leverages its pioneering mRNA platform to rapidly develop and deliver a new class of transformative medicines for a wide range of diseases, fundamentally changing how diseases are treated.
Value Proposition Components
- Component:
Speed & Agility
Clarity:Clear
Uniqueness:Unique
Examples
Our platform enables rapid design, research and testing of multiple mRNAs, within days.
More medicines, faster.
- Component:
Platform Technology
Clarity:Clear
Uniqueness:Somewhat Unique
Examples
We believe that if mRNA can treat one disease, it can treat many diseases.
Limitless treatments
- Component:
Novel Approach
Clarity:Clear
Uniqueness:Somewhat Unique
Examples
Fighting disease differently
This is where mRNA can prove itself again
- Component:
Human Impact & Culture
Clarity:Somewhat Clear
Uniqueness:Common
Examples
- •
Deliver the greatest possible impact to people
- •
Helping more people
- •
Nurturing each individual to thrive and perform at their peak.
Moderna's messaging effectively differentiates itself from traditional pharmaceutical companies by relentlessly focusing on the platform nature of mRNA and its inherent speed. While competitors like Pfizer/BioNTech also use mRNA, Moderna's brand narrative positions them as the pioneering force that built its entire identity and process around this technology. The message is not just that they make mRNA medicines, but that their entire system is a digitally enabled, rapid-response platform for creating them.
The messaging positions Moderna as a futuristic, science- and technology-driven biotech leader rather than a legacy pharma company. They are the architects of 'the mRNAge'. This positions them as more innovative and agile than larger, more established competitors. The emphasis on pipeline and platform technology is a clear signal to investors and the scientific community that their value extends far beyond their initial COVID-19 vaccine success.
Audience Messaging
Target Personas
- Persona:
Potential Employees (Scientists, Tech & Business Professionals)
Tailored Messages
- •
Join us and change the world of medicine
- •
If you’re energized by that kind of environment, you’ll discover new strengths and new ways to grow.
- •
People feel recognized not only for meeting KPIs, but for offering ideas and insights.
Effectiveness:Effective
- Persona:
Investors & Shareholders
Tailored Messages
- •
IR Insights: Recapping Our Second Quarter 2025 Earnings Report
- •
45 Development programs
- •
Our journey to building the best version of Moderna
Effectiveness:Somewhat Effective
- Persona:
General Public & Patients
Tailored Messages
- •
So, what is mRNA?
- •
mRNA medicines fight diseases in a different way than traditional medicine
- •
Helping more people
Effectiveness:Somewhat Effective
- Persona:
Healthcare Professionals & Scientific Community
Tailored Messages
- •
View press releases
- •
Learn more about mRNA
- •
Marta focuses on how Moderna’s field medical teams operate.
Effectiveness:Somewhat Effective
Audience Pain Points Addressed
- •
Lack of treatment options for certain diseases ('desperate need of new options').
- •
Slow pace of traditional drug development ('More medicines, faster').
- •
Desire for a career with purpose and impact ('seeking meaningful impact').
Audience Aspirations Addressed
- •
Hope for a future with cures for more diseases ('Limitless treatments').
- •
Desire to be part of an innovative, world-changing company ('change the world of medicine').
- •
Ambition to work in a dynamic, empowering, and high-growth environment.
Persuasion Elements
Emotional Appeals
- Appeal Type:
Hope
Effectiveness:High
Examples
Limitless treatments
This is where mRNA can prove itself again and potentially make a difference for patients in desperate need of new options.
- Appeal Type:
Purpose / Belonging
Effectiveness:High
Examples
Join us and change the world of medicine
I wanted to help shape how things are done, and Moderna felt like the kind of place where that was possible.
Social Proof Elements
- Proof Type:
Employee Testimonials & Stories
Impact:Strong
Examples
'Meet Keke' section
Marta's Story blog post
- Proof Type:
Data & Metrics
Impact:Moderate
Examples
- •
13+ Years of progress
- •
45 Development programs
- •
36 Ongoing clinical trials
Trust Indicators
- •
Prominent links to press releases and earnings reports.
- •
Clear, simplified explanations of complex science ('So, what is mRNA?').
- •
Publication of corporate responsibility documents ('2024 Impacting Human Health Report').
Scarcity Urgency Tactics
None observed, which is appropriate for this type of corporate website.
Calls To Action
Primary Ctas
- Text:
Learn more about mRNA
Location:Homepage 'So, what is mRNA?' section
Clarity:Clear
- Text:
View all job opportunities
Location:Homepage 'Job Opportunities' section
Clarity:Clear
- Text:
MEET MORE TEAM MEMBERS
Location:Homepage, after employee spotlight
Clarity:Clear
- Text:
Learn more about us
Location:Homepage 'Meet Moderna' section
Clarity:Clear
The CTAs are clear and logically placed, but they are uniformly low-commitment, focused on exploration ('Learn more', 'View'). This is suitable for a corporate branding and informational site. However, there's an opportunity to create more engaging, benefit-oriented CTAs, such as 'Explore our pipeline' or 'Discover a career with impact', to better align with audience motivations.
Messaging Gaps Analysis
Critical Gaps
- •
The patient voice is largely absent. While the company's mission is patient-focused, there are no patient stories or direct messages that illustrate the real-world impact of their science on individuals.
- •
The investor-focused message could be stronger. Beyond linking to earnings reports, the homepage doesn't have a clear, concise narrative articulating why Moderna is a compelling investment.
- •
There is limited messaging that directly addresses potential public skepticism or misinformation about mRNA technology, a crucial aspect of post-pandemic communication.
Contradiction Points
No direct contradictions were found. The messaging is well-aligned across different sections.
Underdeveloped Areas
Pipeline Storytelling: The metric '45 Development programs' is impactful but lacks narrative. The site could do more to tell the story behind these programs, especially in key areas like oncology and rare diseases mentioned in the blog.
Partnership Messaging: The crucial role of partnerships in biotech is not highlighted in the primary site messaging, despite being a key part of their strategy.
Messaging Quality
Strengths
- •
Excellent at explaining complex science in a simple, accessible way ('So, what is mRNA?').
- •
Effectively uses employee stories to humanize the brand and communicate company culture, which is a powerful tool for recruitment.
- •
Strong and consistent articulation of the core value proposition around the mRNA platform's speed and breadth.
Weaknesses
- •
The homepage information hierarchy is not optimized to guide the user through a cohesive narrative.
- •
Over-reliance on generic 'Learn more' CTAs, missing opportunities for more engaging, specific calls to action.
- •
Messaging for patient and investor audiences is less developed compared to the strong messaging for potential employees and the general public.
Opportunities
- •
Develop a dedicated section or content stream for patient-centric stories to emotionally connect the science to its human impact.
- •
Create a more compelling and consolidated narrative for the investor audience, highlighting market opportunities and pipeline milestones.
- •
Use more visual storytelling (e.g., infographics, video) to illustrate the mRNA platform and the development pipeline.
Optimization Roadmap
Priority Improvements
- Area:
Homepage Message Hierarchy
Recommendation:Restructure the homepage to tell a more linear story. Start with the core mission and the transformative power of mRNA, then flow into the pipeline/proof points, then the people/culture, and finally the corporate/news elements.
Expected Impact:High
- Area:
Patient-Centric Content
Recommendation:Launch a content initiative focused on the patient journey and the diseases Moderna aims to treat. This could include articles, videos, and testimonials (where regulations permit).
Expected Impact:High
- Area:
Call-to-Action Language
Recommendation:Revise generic CTAs to be more specific and benefit-driven. For example, change 'Learn more' under the mRNA section to 'Explore our science' or 'See how mRNA works'.
Expected Impact:Medium
Quick Wins
- •
Make the pipeline metrics ('45 Development programs') a clickable link that leads to a more detailed, visually engaging pipeline page.
- •
Add a short, compelling headline to the 'Latest News' section that frames the news within the company's broader mission.
- •
Incorporate the 'Change the world of medicine' tagline more prominently near the primary mission statement on the homepage.
Long Term Recommendations
- •
Develop distinct messaging tracks or user journeys on the website for key audiences (e.g., 'For Investors', 'For Patients', 'For Scientists').
- •
Invest in interactive content that allows users to explore the mRNA platform and its potential applications in a more dynamic way.
- •
Build out a thought leadership platform that goes beyond company news to discuss the future of medicine, biotechnology, and public health, solidifying their position as industry visionaries.
Moderna's strategic messaging is fundamentally strong, built on a clear and compelling value proposition centered on the revolutionary potential of its mRNA platform. The brand voice is consistently ambitious, scientific, and increasingly human-centric, effectively communicating its position as a pioneering force in biotechnology. The website excels at simplifying complex science for a broad audience and leverages employee stories to build a powerful employer brand. However, the overall effectiveness is hampered by a fragmented message hierarchy on the homepage, which dilutes the primary narrative by giving equal weight to disparate corporate functions like investor relations, news, and recruitment without a unifying thread. The messaging is most highly developed for potential employees and the general public, but there are significant gaps in content tailored to patients and a less-than-compelling narrative for investors. To elevate its strategic impact, Moderna should refine its site architecture to tell a more cohesive story, deepen its patient-centric content, and sharpen its calls to action to better guide and engage its diverse audiences.
Growth Readiness
Growth Foundation
Product Market Fit
Moderate
Evidence
- •
Strong, demonstrated PMF for COVID-19 vaccine (Spikevax), establishing credibility and generating significant cash reserves.
- •
Emerging PMF for RSV vaccine (mRESVIA), with recent FDA approvals, but facing stiff competition from established players like Pfizer and GSK.
- •
Vast therapeutic pipeline with 45 development programs, including several in late-stage (Phase 3) trials for oncology, rare diseases, and other vaccines, indicating potential for future PMF in new markets.
Improvement Areas
- •
Accelerate clinical trials and secure regulatory approvals for late-stage pipeline candidates (e.g., combo flu/COVID vaccine, CMV vaccine, personalized cancer vaccine).
- •
Successfully commercialize non-COVID products to prove the platform's versatility and diversify revenue streams away from the declining COVID market.
- •
Demonstrate superior efficacy or logistical advantages (e.g., stability, dosing) for new products like the RSV vaccine to capture market share from entrenched competitors.
Market Dynamics
High (mRNA Therapeutics market projected CAGR of 16.8% - 24.78% from 2025-2030).
Growing
Market Trends
- Trend:
Expansion Beyond Infectious Diseases
Business Impact:Significant opportunity to leverage the mRNA platform for high-value areas like oncology, rare diseases, and personalized medicine, moving beyond the commoditizing vaccine market.
- Trend:
AI and Machine Learning in Drug Discovery
Business Impact:Ability to accelerate R&D timelines, optimize clinical trial design, and discover novel therapies faster. Moderna is actively using AI to gain a competitive edge.
- Trend:
Increased Competition in mRNA
Business Impact:Direct competition from players like Pfizer/BioNTech and established pharma giants entering the space requires continuous innovation and strong commercial execution to maintain leadership.
- Trend:
Focus on Combination Vaccines
Business Impact:Strong market demand for convenience creates a significant opportunity for combo vaccines (e.g., Flu/COVID), where Moderna has a potential first-mover advantage.
Pivotal. While the initial COVID-19 wave has passed, the market is primed for the next generation of mRNA medicines. Moderna's ability to execute on its pipeline in the next 2-3 years is critical to capture this opportunity.
Business Model Scalability
High
High fixed costs associated with R&D, clinical trials, and manufacturing facilities. Variable costs per dose are relatively low, allowing for high gross margins on successful, scaled products.
High potential for operational leverage once a drug is approved and commercialized, as the core mRNA platform can be reused for different targets, reducing incremental R&D time.
Scalability Constraints
- •
Navigating complex, country-specific regulatory approval processes for each new product.
- •
Scaling manufacturing for a diverse portfolio of products with different dosage and delivery requirements.
- •
Securing market access and reimbursement agreements with global payers, a different challenge than government pandemic contracts.
Team Readiness
Proven. The leadership team successfully navigated the intense pressure of developing and scaling a pandemic vaccine. The current challenge is transitioning from a crisis-response company to a multi-product commercial biotech.
Transitioning. The company is undergoing restructuring and cost-cutting to align with post-pandemic realities, including a 10% workforce reduction. This signals a necessary shift towards a more sustainable operational model.
Key Capability Gaps
- •
Commercialization expertise in therapeutic areas beyond vaccines, particularly in oncology and rare diseases.
- •
Health Economics and Outcomes Research (HEOR) capabilities to build value propositions for payers and secure favorable pricing.
- •
Global market access and reimbursement negotiation skills for a competitive, non-pandemic environment.
Growth Engine
Commercialization Channels
- Channel:
Government Contracts (Pandemic Preparedness)
Effectiveness:High
Optimization Potential:Medium
Recommendation:Maintain strong relationships with governments (e.g., BARDA, UK) for future pandemic preparedness contracts and stockpile opportunities.
- Channel:
Direct Sales to Healthcare Systems & Pharmacy Chains
Effectiveness:Medium
Optimization Potential:High
Recommendation:Build a world-class commercial sales force to compete with Pfizer and GSK for seasonal vaccine contracts (RSV, Flu), focusing on product differentiation and supply chain reliability.
- Channel:
Pharmaceutical Partnerships (Co-development/Co-commercialization)
Effectiveness:High
Optimization Potential:High
Recommendation:Continue leveraging partnerships, like the one with Merck for the personalized cancer vaccine, to de-risk development and access established commercial infrastructure in new therapeutic areas.
Customer Journey
For therapeutics, the 'customer journey' is the clinical development and regulatory approval pathway. It involves successful trial recruitment, positive data readouts, regulatory submission, payer negotiation, and finally physician prescription.
Friction Points
- •
Phase 3 clinical trial failures or delays, which can erase billions in potential value.
- •
Securing broad reimbursement from insurance companies and national health systems.
- •
Convincing physicians to adopt a new therapeutic modality over the existing standard of care.
Journey Enhancement Priorities
{'area': 'Clinical Trial Optimization', 'recommendation': 'Use AI and real-world data to optimize patient selection, accelerate recruitment, and improve the probability of trial success.'}
{'area': 'Payer Value Proposition', 'recommendation': 'Develop compelling health economic data early in Phase 3 to demonstrate the value of pipeline drugs to payers, facilitating smoother reimbursement negotiations.'}
Retention Mechanisms
- Mechanism:
Product Efficacy & Safety Profile
Effectiveness:High
Improvement Opportunity:Continuously improve the mRNA platform to enhance efficacy and reduce side effects, making new products the clear standard of care.
- Mechanism:
Combination Products
Effectiveness:Emerging
Improvement Opportunity:Successfully launching a combo Flu/COVID vaccine would create significant customer stickiness due to convenience, locking in market share for both components.
- Mechanism:
Platform Leadership
Effectiveness:High
Improvement Opportunity:Maintain a cadence of innovation, launching new products that address unmet needs, which reinforces Moderna's brand as the leader in mRNA medicine.
Revenue Economics
Challenging (Transition Phase). Currently experiencing significant net losses ($825M in Q2 2025) as high R&D spending continues while COVID revenue declines sharply. The model is predicated on future blockbuster approvals to cover these costs.
Not Applicable (Replaced with Pipeline Potential vs. R&D Investment)
Low (Current). The company is in a planned investment phase. Revenue for 2025 is projected at $1.5B-$2.5B while operating expenses are projected to be significantly higher. Breakeven is targeted for 2028.
Optimization Recommendations
- •
Execute disciplined portfolio prioritization, discontinuing or pausing lower-probability programs to focus R&D investment on late-stage assets with the highest potential return.
- •
Aggressively manage operating expenses and cost of goods to preserve cash reserves ($7.5B at end of Q2 2025) until new revenue streams come online.
- •
Secure non-dilutive funding through strategic partnerships and government grants to offset R&D costs.
Scale Barriers
Technical Limitations
- Limitation:
mRNA Delivery to Specific Tissues
Impact:High
Solution Approach:Current LNP technology primarily targets the liver. Developing novel delivery systems (e.g., new LNPs, polymers) to target other organs is critical for expanding therapeutic applications beyond vaccines and some liver-targeted therapies.
- Limitation:
Immunogenicity with Chronic Dosing
Impact:High
Solution Approach:For therapies requiring repeated administration (e.g., for rare genetic diseases), the immune system's reaction to foreign mRNA is a major hurdle. Research into next-generation RNA modifications (e.g., circRNA) and delivery vehicles is needed to enable long-term treatment.
- Limitation:
Product Thermostability
Impact:Medium
Solution Approach:While improved, cold-chain requirements for mRNA products can be a logistical barrier. Continued R&D into more stable formulations (e.g., lyophilized powders) will provide a competitive advantage, especially in emerging markets.
Operational Bottlenecks
- Bottleneck:
Multi-Product Manufacturing & Fill-Finish
Growth Impact:Transitioning from single-product (Spikevax) to multi-product manufacturing creates complexity in scheduling, quality control, and supply chain. Securing dedicated fill-finish capacity is crucial.
Resolution Strategy:Leverage long-term strategic manufacturing collaborations (e.g., with Thermo Fisher Scientific) and build out dedicated, flexible facilities (e.g., in the UK and Canada) to handle a diverse pipeline.
- Bottleneck:
Global Clinical Trial Execution
Growth Impact:Running multiple simultaneous Phase 3 global trials is immensely complex and expensive. Delays in recruitment or execution can push back revenue-generating approvals by years.
Resolution Strategy:Invest in a world-class clinical operations team and leverage technology (AI, decentralized trials) to streamline trial management and accelerate timelines.
Market Penetration Challenges
- Challenge:
Intense Competition from Established Pharma
Severity:Critical
Mitigation Strategy:Compete on scientific innovation by demonstrating superior clinical data. For commercial execution, be nimble and potentially partner in areas where competitors (Pfizer, GSK, Merck) have established dominance.
- Challenge:
Post-Pandemic Market Dynamics
Severity:Major
Mitigation Strategy:Shift focus from a pandemic sales model (government contracts) to a traditional commercial model, which requires building strong relationships with payers, physicians, and pharmacy benefit managers. This includes overcoming a consumer preference for Pfizer's COVID vaccine in some surveys.
- Challenge:
Pricing & Reimbursement Pressure
Severity:Major
Mitigation Strategy:Develop robust pharmacoeconomic models and real-world evidence to justify pricing for novel therapies, especially high-cost treatments for cancer and rare diseases.
Resource Limitations
Talent Gaps
- •
Experienced commercial launch leaders for oncology and rare disease.
- •
Regulatory affairs specialists with deep expertise in non-vaccine therapeutic areas.
- •
Experts in health economics and market access.
High. While the company has a strong cash position ($7.5B), the burn rate is significant due to massive R&D costs. Continued access to capital markets or partnership funding may be necessary to fully fund the pipeline to commercialization.
Infrastructure Needs
- •
Expansion of global manufacturing network to support a multi-product portfolio.
- •
Investment in commercial and medical affairs infrastructure in key international markets.
- •
Robust digital infrastructure to leverage AI across R&D and commercial operations.
Growth Opportunities
Market Expansion
- Expansion Vector:
New Therapeutic Areas (Oncology)
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Co-develop and co-commercialize the personalized cancer vaccine (mRNA-4157) with Merck to leverage their oncology expertise and global footprint, de-risking market entry.
- Expansion Vector:
Geographic Expansion for New Products
Potential Impact:High
Implementation Complexity:Medium
Recommended Approach:Establish a sequential launch strategy for new products like mRESVIA and the flu vaccine, prioritizing markets with clear regulatory pathways and favorable reimbursement landscapes (e.g., US, EU, Japan).
Product Opportunities
- Opportunity:
Combination Respiratory Vaccines (Flu + COVID)
Market Demand Evidence:Strong preference for convenience from both consumers and healthcare systems. Represents a >$10B potential market.
Strategic Fit:Directly leverages core expertise in respiratory vaccines and manufacturing scale. A successful combo product (mRNA-1083) could secure a dominant market position.
Development Recommendation:Prioritize completion of Phase 3 trials and regulatory submissions to be first-to-market or best-in-class.
- Opportunity:
Personalized Cancer Vaccines (PCV)
Market Demand Evidence:Significant unmet need in treating cancers like melanoma and non-small cell lung cancer. Early data is promising.
Strategic Fit:Represents the ultimate potential of the mRNA platform for personalized medicine, creating a massive competitive moat if successful.
Development Recommendation:Execute flawlessly on the Phase 3 trials with Merck and build out the complex manufacturing and logistics required for an individualized therapy.
- Opportunity:
Latent Virus Vaccines (e.g., CMV, EBV)
Market Demand Evidence:Large, untapped markets with significant unmet medical needs. The CMV vaccine (mRNA-1647) is in Phase 3.
Strategic Fit:Expands the vaccine portfolio beyond seasonal respiratory viruses into new, durable markets.
Development Recommendation:Continue advancing the CMV vaccine towards approval and progress the EBV and HSV candidates through mid-stage trials.
Channel Diversification
- Channel:
Direct-to-Payer Contracting
Fit Assessment:Excellent
Implementation Strategy:Build a dedicated market access team to negotiate value-based contracts and formulary placement directly with large insurance companies and national health systems.
Strategic Partnerships
- Partnership Type:
Co-Development & Commercialization
Potential Partners
Merck (existing)
Other large pharma with established infrastructure in oncology, cardiology, or immunology.
Expected Benefits:Share high R&D costs, mitigate risk, and access deep therapeutic area expertise and global commercialization channels.
- Partnership Type:
Technology & AI Collaboration
Potential Partners
- •
IBM (existing)
- •
NVIDIA
- •
Leading AI-native drug discovery companies.
Expected Benefits:Accelerate drug discovery, improve delivery systems, and optimize manufacturing processes by integrating cutting-edge AI and quantum computing technologies.
- Partnership Type:
Manufacturing & Supply Chain
Potential Partners
- •
Thermo Fisher Scientific (existing)
- •
Lonza (existing)
- •
Regional contract manufacturing organizations (CMOs).
Expected Benefits:Ensure robust, scalable, and geographically diverse manufacturing capacity for a global multi-product portfolio, enhancing supply chain resilience.
Growth Strategy
North Star Metric
Number of Late-Stage (Phase 3) Pipeline Programs
For a platform company in its current stage, the most critical indicator of future growth and value is the successful progression of its pipeline. This metric directly reflects the conversion of R&D investment into tangible, near-commercial assets.
Advance at least 2-3 new programs into Phase 3 trials annually over the next three years.
Growth Model
Platform-Led Growth
Key Drivers
- •
Repeatable success of the core mRNA technology platform across different diseases.
- •
Speed of transition from discovery to clinical trials.
- •
Successful negotiation of the regulatory and reimbursement gauntlet for each new product.
Focus investment on strengthening the core platform (delivery, manufacturing, AI) while simultaneously running a portfolio of therapeutic 'bets'. Systematically apply learnings from each program to accelerate all others.
Prioritized Initiatives
- Initiative:
Achieve Regulatory Approval for a Next-Generation Respiratory Vaccine (e.g., Flu/COVID combo)
Expected Impact:High
Implementation Effort:High
Timeframe:12-24 months
First Steps:Finalize Phase 3 trial data analysis and prepare comprehensive regulatory submission packages for the FDA and EMA.
- Initiative:
Successfully Execute Phase 3 Trials for Personalized Cancer Vaccine (mRNA-4157)
Expected Impact:High
Implementation Effort:High
Timeframe:24-36 months
First Steps:Work with partner Merck to ensure optimal trial execution, patient recruitment, and development of the bespoke manufacturing process required for a personalized therapy.
- Initiative:
Execute on Cost Optimization and Operational Efficiency Plan
Expected Impact:Medium (Financial Health)
Implementation Effort:Medium
Timeframe:Ongoing to 2028
First Steps:Implement the announced workforce restructuring and conduct a rigorous review of all non-pipeline R&D and SG&A spending to achieve targeted savings and extend the cash runway.
Experimentation Plan
High Leverage Tests
- Test:
Adaptive Clinical Trial Designs
Hypothesis:Using adaptive designs in Phase 2/3 trials can accelerate timelines and increase the probability of success by allowing for modifications based on interim data.
Area:Clinical Development
- Test:
AI-Powered Biomarker Discovery
Hypothesis:Leveraging AI to analyze genomic and patient data can identify novel biomarkers to better target patient populations for therapies like the PCV.
Area:R&D / Personalized Medicine
Clinical trial success rates, time-to-approval, patient-reported outcomes, and ultimately, prescription volume and market share for commercialized products.
Aligned with the structured phases of clinical drug development (pre-clinical, Phase 1, 2, 3).
Growth Team
Franchise-based model aligned to key therapeutic areas (Respiratory Vaccines, Oncology, Rare Diseases, Latent Vaccines). Each franchise should have dedicated leadership for clinical, regulatory, and commercial functions, supported by a centralized Platform R&D and operations team.
Key Roles
- •
General Manager, Oncology Franchise
- •
Global Head of Market Access & Pricing
- •
Chief Commercial Officer
- •
Head of Clinical Operations
Aggressively hire senior commercial and market access talent from established large-cap pharmaceutical companies. Foster a culture that blends the speed and innovation of biotech with the disciplined execution of big pharma.
Moderna is at a critical inflection point, transitioning from a single-product pandemic hero to a diversified, multi-product biotechnology company. The company's core growth foundation is its powerful and validated mRNA platform, which provides high business model scalability and excellent market timing in a rapidly growing industry. However, its current product-market fit is moderate, as it relies heavily on a declining COVID-19 vaccine market and is only beginning to commercialize new products against fierce competition.
The primary growth engine has shifted from pandemic response to a deliberate, R&D-driven pipeline. Success hinges on converting this extensive pipeline—particularly late-stage assets in combination respiratory vaccines, oncology, and latent viruses—into commercial revenue streams. This requires overcoming significant scale barriers, including intense competition from established pharmaceutical giants, navigating complex global regulatory and reimbursement landscapes, and solving technical challenges related to drug delivery and chronic dosing.
Key growth opportunities are immense and lie in successfully launching products in new, high-value therapeutic areas. The personalized cancer vaccine with Merck and the combination Flu/COVID vaccine are standout opportunities that could redefine the company's future.
To achieve this, the recommended growth strategy is a 'Platform-Led' model focused on a North Star Metric of increasing the 'Number of Late-Stage (Phase 3) Pipeline Programs.' The immediate priorities are securing approval for a next-generation respiratory vaccine, flawlessly executing the pivotal oncology trials, and maintaining strict financial discipline to extend the cash runway until breakeven, targeted for 2028. Building out commercial and market access capabilities is as critical as scientific innovation for Moderna's next chapter of growth.
Legal Compliance
Moderna's Privacy Statement is comprehensive, reflecting its status as a global biotechnology company. It explicitly addresses data collection from various sources, including voluntary provisions (e.g., website visits, newsletter sign-ups), healthcare professionals (HCPs), and automated tools like cookies. It correctly identifies legal bases for processing under GDPR, such as legitimate business purposes, consent, legal obligations (especially for pharmacovigilance), and contractual necessity. The policy details data sharing practices with service providers and outlines procedures for international data transfers, citing the use of Standard Contractual Clauses (SCCs) for transfers outside the EU/EEA, which is a key GDPR requirement. Specific sections are dedicated to the rights of individuals in the EU/EEA, UK, and Switzerland, including rights to access, correct, or delete personal data, demonstrating a clear attempt to comply with regional data protection laws. The policy also covers data retention, security measures, and adverse event reporting obligations to regulatory bodies like the FDA and EMA.
The Terms and Conditions of Use are present but appear dated ('2018-11-09'), which is a potential concern given the company's significant evolution since then. The terms cover key areas such as intellectual property (protecting trademarks), permitted use of website information (non-commercial), and disclaimers of liability. A critical clause states, 'Any information submitted or provided by you to the Website might be publicly accessible. Important and private information should be protected by you,' which is a broad and potentially problematic disclaimer that may not be fully enforceable, especially concerning personal data governed by privacy laws. The choice of law is designated as The Commonwealth of Massachusetts, which provides legal clarity. However, the overall document lacks the specificity and robustness expected for a company of Moderna's scale and risk profile, particularly regarding user-generated content, account responsibilities, and dispute resolution mechanisms.
Moderna provides a detailed Cookie Statement that explains the use of cookies, pixel tags, and similar technologies. It categorizes cookies into types like 'essential', 'functionality', and 'analytics' and identifies third-party services used, such as Adobe Analytics, Google Analytics, Marketo, and Qualtrics. The statement clarifies that while cookies themselves do not collect PII, the data collected can be combined with other personal data, which then falls under their main Privacy Statement. It provides links to opt-out of certain analytics services. However, the compliance strength heavily depends on the front-end user experience of the cookie consent banner itself (which cannot be fully analyzed from the provided text). A key compliance point is whether the banner provides clear 'accept' and 'reject' options with equal prominence and avoids pre-ticked boxes for non-essential cookies, which is a stringent requirement under GDPR.
Moderna's data protection framework, as articulated in its privacy policy, is robust and tailored to a global audience. The company has a clear understanding of its obligations under GDPR, as evidenced by its discussion of legal bases for processing, data subject rights, and international data transfer mechanisms (SCCs). With a significant commercial presence across Europe, including subsidiaries in major EU countries and a European headquarters in Switzerland, GDPR compliance is non-negotiable. The policy also mentions CCPA, indicating awareness of US state-level privacy laws, which is relevant given their planned expansion in California. The appointment of an EU DPO contact ([email protected]) is a direct compliance measure under GDPR. The policy's detailed section on pharmacovigilance highlights the lawful processing of sensitive health data to comply with public health and safety regulations, a critical aspect for a pharmaceutical company.
The provided website data shows positive indicators for accessibility compliance. The inclusion of a 'Skip to main content' link at the top of the pages is a fundamental and important feature for users who rely on keyboard navigation or screen readers. [https://www.modernatx.com/en-US/media-center/all-media/blogs/building-systems-with-purpose#main, https://www.modernatx.com/en-US#main] This demonstrates a foundational awareness of WCAG (Web Content Accessibility Guidelines) principles. A full analysis would require automated testing and manual review of the live site for aspects like color contrast, ARIA roles, image alt-text, and keyboard trap issues. However, the initial evidence suggests a commitment to basic accessibility, which is crucial for a company focused on public health and serving a diverse global audience.
As a publicly-traded biotechnology company, Moderna is subject to a complex web of industry-specific regulations from the SEC, FDA (in the U.S.), and EMA (in the EU). The website content, particularly press releases and blogs, must carefully navigate these rules.
1. SEC Compliance (Forward-Looking Statements): Press releases on the website include a 'Forward-Looking Statements' section with a safe harbor provision, as required by the Private Securities Litigation Reform Act of 1995. This is critical for managing investor expectations and mitigating liability for projections about clinical trials, regulatory approvals, and financial performance. These statements are designed to be accompanied by meaningful cautionary language identifying risk factors.
2. FDA/EMA Marketing & Promotion: The language used on the website appears carefully controlled to avoid making unsubstantiated claims about pipeline products or engaging in off-label promotion. Per FDA and EMA regulations, advertising for prescription drugs is heavily restricted and must be balanced, truthful, and non-misleading. The website focuses on corporate mission, mRNA science, and employee stories rather than direct-to-consumer (DTC) advertising of specific pipeline products, which aligns with compliance best practices.
3. Pharmacovigilance: The privacy policy correctly identifies the legal obligation to collect and report information on adverse events, which is a critical regulatory function for any drug manufacturer.
Compliance Gaps
- •
The 'Terms and Conditions of Use' document is dated '2018-11-09', which predates the company's massive global expansion and the launch of its primary commercial product; it likely requires a comprehensive update.
- •
The disclaimer in the Terms of Use stating that user-submitted information 'might be publicly accessible' is overly broad and could conflict with data protection obligations under GDPR/CCPA if personal data is submitted.
- •
Lack of a visible, dedicated section or link for 'Forward-Looking Statements' or 'Investor Relations Disclaimers' in the main website footer, which is a common best practice for publicly-traded companies.
- •
Absence of clear, easily accessible information for healthcare professionals regarding adverse event reporting directly on the main website pages (though it is mentioned within the privacy policy).
Compliance Strengths
- •
A detailed and geographically aware Privacy Statement that addresses GDPR and CCPA requirements, including legal bases for processing and data subject rights.
- •
Clear mechanisms for international data transfers (SCCs) are disclosed, reflecting a mature approach to global data protection.
- •
Proper use of 'safe harbor' provisions for forward-looking statements in press releases, demonstrating strong compliance with SEC regulations.
- •
Website content appears to be carefully curated to align with FDA/EMA restrictions on pharmaceutical marketing, focusing on science and corporate information rather than prohibited product promotion.
- •
Implementation of basic but important accessibility features like 'Skip to main content' links.
Risk Assessment
- Risk Area:
Outdated Terms of Service
Severity:Medium
Recommendation:Conduct a comprehensive review and update of the Terms and Conditions of Use to reflect the company's current global scale, commercial status, and risk profile. Pay special attention to clauses on liability, user data, dispute resolution, and intellectual property.
- Risk Area:
FDA/EMA Advertising Violations
Severity:High
Recommendation:Maintain and continuously audit all public-facing content (blogs, press releases, social media) to ensure it does not implicitly or explicitly make unapproved claims about investigational products or engage in off-label promotion. All marketing and communications materials should undergo rigorous legal and regulatory review.
- Risk Area:
Insufficient Cookie Consent Mechanism
Severity:Medium
Recommendation:Ensure the website's cookie consent banner meets the highest standards of GDPR: provide equally prominent 'accept' and 'reject' options on the first layer, do not use pre-ticked boxes for non-essential cookies, and provide granular controls for users.
- Risk Area:
SEC Disclosure Violations
Severity:High
Recommendation:Ensure all forward-looking statements are consistently identified and accompanied by specific, meaningful cautionary language detailing risk factors. Avoid boilerplate warnings. Make investor-related disclaimers easily accessible from the website footer.
- Risk Area:
Data Breach / Privacy Violation
Severity:High
Recommendation:Regularly review and update the Privacy Statement to align with new global regulations. Conduct periodic data protection impact assessments (DPIAs) for new processing activities and ensure robust security measures are in place to protect sensitive health and personal data.
High Priority Recommendations
- •
Immediately initiate a legal review to update the 'Terms and Conditions of Use' to reflect the company's post-2018 transformation into a global commercial entity.
- •
Audit the website's cookie consent implementation to confirm strict compliance with GDPR's consent requirements (e.g., explicit 'reject' option).
- •
Enhance the website footer to include direct links to 'Investor Disclaimers' and 'Forward-Looking Statements' to improve transparency and bolster SEC safe harbor compliance.
- •
Create a more prominent and easily accessible pathway for healthcare professionals and patients to report adverse events, beyond its mention in the privacy policy.
Moderna's legal positioning reflects a high degree of sophistication, particularly in the critical areas of data privacy and regulatory compliance for securities and pharmaceutical marketing. The company's global presence in the EU, USA, and other regions is matched by a detailed Privacy Statement that is clearly aligned with the stringent requirements of GDPR and aware of CCPA. This robust data protection framework is a strategic asset, building trust with patients, healthcare providers, and partners in a data-sensitive industry.
From an industry-specific perspective, Moderna demonstrates strong adherence to SEC and FDA/EMA regulations. The careful use of 'safe harbor' statements in financial communications and the disciplined, science-focused content on its public website mitigates significant legal and financial risks associated with investor disclosures and off-label promotion. This compliance posture is essential for maintaining market access and credibility with regulators.
However, there are notable gaps in foundational legal documentation, specifically the outdated Terms of Service. This document from 2018 does not adequately represent the company's current operational scale and risk landscape. This discrepancy, while seemingly minor, creates unnecessary legal ambiguity. Overall, Moderna's legal positioning is strong in its most high-risk, industry-specific domains, but it requires administrative updates to its general website legal framework to achieve comprehensive excellence.
Visual
Design System
Corporate & Scientific
Excellent
Advanced
User Experience
Navigation
Horizontal Top Bar with Audience-Specific Sub-Navigation
Intuitive
Good
Information Architecture
Logical
Clear
Light
Conversion Elements
- Element:
CTA Links (e.g., 'Learn More', 'Read Our Blog')
Prominence:Low
Effectiveness:Somewhat Ineffective
Improvement:Redesign primary and secondary CTAs to use button styles (e.g., solid fill for primary, outline/ghost for secondary). This will increase their visual weight, improve scannability, and create a clearer action hierarchy, likely boosting engagement with key content funnels.
- Element:
Recruitment Funnel (Job Listings)
Prominence:Medium
Effectiveness:Effective
Improvement:On the main careers page, implement more prominent and advanced filtering options (e.g., by department, location, keyword) to improve the user experience for prospective candidates navigating a large number of open roles.
- Element:
Investor & Media Portals
Prominence:High
Effectiveness:Effective
Improvement:Ensure the landing pages for these portals use clear, large-font cards or links to direct users immediately to the most frequently sought-after documents, such as the latest earnings report, press kits, or SEC filings.
Assessment
Strengths
- Aspect:
Clean, Trustworthy Aesthetic
Impact:High
Description:The design employs ample white space, a structured grid, and high-quality, professional imagery. This creates a polished, credible, and scientific aesthetic that is critical for building trust with investors, healthcare professionals, and the public in the biotechnology industry.
- Aspect:
Audience-Centric Information Architecture
Impact:High
Description:The website's structure and navigation are logically organized to serve multiple, distinct audiences (e.g., Investors, Healthcare Professionals, Media, Job Seekers). This clear segmentation allows different user personas to find relevant information efficiently, which is a key strength for a multifaceted corporate site.
- Aspect:
Consistent Brand Identity Expression
Impact:Medium
Description:The consistent use of the Moderna logo, the specific red and blue color palette, and a clean sans-serif typography suite reinforces a strong, unified brand identity. This consistency across pages builds brand recognition and conveys professionalism.
Weaknesses
- Aspect:
Low-Affordance Calls-to-Action
Impact:High
Description:Key CTAs are styled as simple text links with an arrow icon. They lack visual prominence and fail to stand out from non-interactive text, creating low affordance. This subtlety can lead to lower click-through rates on critical user journeys, such as exploring the pipeline or reading key reports.
- Aspect:
Overly Conventional Content Presentation
Impact:Medium
Description:While professional, the page layouts are very traditional and static. For a company at the forefront of mRNA technology, the design doesn't fully express this spirit of innovation. There is a missed opportunity to use more dynamic or interactive elements to explain complex science and tell the company's story.
- Aspect:
Lack of Visual Storytelling
Impact:Medium
Description:The reliance on corporate stock-style photography is functional but doesn't build a strong emotional connection. The 'Building Systems with a Purpose' blog post is a step in the right direction, but the overall site could benefit from more human-centric stories, custom illustrations, or infographics to make the science and company culture more accessible and engaging.
Priority Recommendations
- Recommendation:
Redesign and A/B Test Call-to-Action Buttons
Effort Level:Low
Impact Potential:High
Rationale:Introduce a clear button hierarchy (primary, secondary, tertiary). Use a solid red button for the most important actions to significantly increase their visibility and clickability. This is a low-effort change that can directly improve user flow completion and engagement with key business objectives.
- Recommendation:
Develop Interactive 'mRNA Explained' Module
Effort Level:High
Impact Potential:High
Rationale:To truly own the 'mRNAge' and communicate innovation, create an interactive, visually rich module that explains how the technology works. Using animations, scrollytelling, and simplified diagrams will demystify the science, increase user dwell time, and strongly differentiate the brand from competitors. This would be a cornerstone content piece.
- Recommendation:
Diversify Visual Assets Beyond Photography
Effort Level:Medium
Impact Potential:Medium
Rationale:Commission a suite of custom illustrations and data visualizations that align with the brand's color palette. These assets can be used to explain complex topics, break up text-heavy pages, and add a unique, ownable visual language that enhances the brand's identity as a scientific innovator.
Mobile Responsiveness
Good
The component-based card design and simple grid layout suggest that the site will adapt well to various screen sizes. Typography and spacing appear to be managed effectively, though live testing is required for a definitive assessment.
Mobile Specific Issues
The text-based CTAs may be difficult to accurately tap on smaller touch screens.
Full-width image-and-text blocks will stack vertically, which may create long scrolling experiences on content-heavy pages.
Desktop Specific Issues
Large amounts of white space on ultra-widescreen monitors could cause some layout components to feel disconnected from one another if max-width constraints are not properly set.
Executive Summary
The Moderna website (modernatx.com) projects a highly professional, credible, and trustworthy image, which is essential for its role as a leading biotechnology firm. Its core strengths lie in a mature and consistent design system, a clean corporate aesthetic, and a logical information architecture that effectively caters to its diverse and critical audiences, including investors, healthcare professionals (HCPs), and potential talent. However, the design, while competent, is conservative and fails to fully capture the innovative, cutting-edge nature of Moderna's mRNA technology. The most significant weakness is the uniformly low prominence of its calls-to-action (CTAs), which likely suppresses user engagement and hinders key user journeys. Strategic improvements should focus on enhancing actionability through redesigned CTAs, introducing dynamic visual storytelling to explain complex science, and diversifying visual assets to create a more ownable and engaging brand experience.
Detailed Analysis
1. Design System Coherence and Brand Identity Expression
The site demonstrates an Advanced and highly coherent design system. The brand's color palette—primarily white and gray with a distinct red for accents and CTAs and a secondary blue—is applied consistently. Typography is clean, legible, and hierarchically structured, reinforcing a scientific and professional tone. The overall style is Corporate & Scientific, prioritizing clarity and trustworthiness over expressive design trends. This approach successfully builds brand credibility but leaves room for more visual innovation to match the company's scientific reputation.
2. Visual Hierarchy and Information Architecture
The visual hierarchy is generally effective. Headlines are clearly distinguished, and content is chunked into logical sections using cards and structured layouts, resulting in a Light cognitive load for the user. The information architecture is a key strength, with a primary navigation focused on corporate pillars (The Power of mRNA, Research, Responsibility) and a secondary, audience-focused navigation (Healthcare Professionals, Investors, Media) that provides clear, tailored user flows.
3. Navigation Patterns and User Flow Optimization
The use of a standard Horizontal Top Bar is intuitive and follows established conventions. The inclusion of audience-specific entry points is a best practice for a corporate site with such varied stakeholders. User flows from the homepage to key sections like 'Latest News', 'Job Opportunities', and 'Earnings Report' are clear. The primary friction in user flow is not the structure but the weakness of the CTAs that are meant to guide users along these paths.
4. Mobile Responsiveness and Cross-Device Experience
Based on the fluid grid and component-based design evident in the screenshots, the site's mobile responsiveness is assessed as Good. Content blocks are designed to stack cleanly on smaller viewports. However, the low visual weight of CTAs could be exacerbated on mobile, where tappable elements need to be clear and sufficiently large to avoid user error and frustration.
5. Visual Conversion Elements and Call-to-Action Effectiveness
This is the most significant area for improvement. The site's 'conversion' goals are not e-commerce sales but rather user actions like downloading a report, applying for a job, or reading a press release. The current CTA design—understated text links with an arrow—is Ineffective at drawing the user's eye and compelling action. They blend in with informational links, forcing users to read and scan carefully rather than being guided by visually distinct action cues. Elevating these elements into clear buttons would be a high-impact, low-effort change.
6. Visual Storytelling and Content Presentation
The website successfully tells a story of a professional, serious, and scientifically-grounded company. The blog post about an employee ('Marta's Story') is an excellent example of humanizing the brand. However, this is the exception rather than the rule. The broader narrative about the revolutionary potential of mRNA technology is told through text rather than shown through compelling visuals. There is a significant opportunity to incorporate more dynamic content, such as interactive diagrams, custom illustrations, and video, to make the complex science more accessible and to better reflect the company's innovative spirit.
Discoverability
Market Visibility Assessment
Moderna has established significant brand authority as a pioneer in mRNA technology, largely built on the rapid development and success of its COVID-19 vaccine, Spikevax. The company is recognized as a key player in the biotech industry, synonymous with cutting-edge science. However, its authority is strongly tied to the pandemic, and the current challenge is to extend this perception to its broader pipeline in oncology, rare diseases, and other infectious diseases. Digital content, including the website, focuses heavily on the science of mRNA, its corporate mission, and its expanding pipeline, positioning the company as a forward-looking, science-driven organization rather than just a 'COVID vaccine company'.
In the digital landscape, Moderna's visibility is highest in searches related to 'mRNA vaccines' and 'COVID-19 vaccine'. It faces intense competition for visibility from Pfizer/BioNTech, which often dominates search results due to Pfizer's long-standing market presence and larger marketing infrastructure. In emerging areas like RSV vaccines, Moderna is a new entrant competing against established players like GSK and Pfizer, where it currently has negligible market share and lower digital visibility. Visibility for its oncology and rare disease pipeline is nascent and primarily targeted at investors and the scientific community, not yet capturing broader market awareness.
For Moderna, 'customer acquisition' translates to influencing healthcare providers (HCPs), securing governmental and institutional contracts, attracting top scientific talent, and maintaining investor confidence. The digital presence is a primary channel for these objectives. The website's content on mRNA technology and its pipeline directly supports engagement with HCPs and researchers. The prominent 'Careers' and 'Investor Relations' sections indicate that talent and investor acquisition are key digital goals. The potential is high, as surveys show HCPs are highly familiar with and confident in mRNA technology, creating a receptive audience for new product information.
Digitally, Moderna has a strong North American and European presence, reflective of its primary markets and manufacturing footprint. The website is primarily in English, which may limit direct engagement in non-English speaking regions. Strategic partnerships are key to its international market penetration. Expansion into new geographic markets, such as the 2023 agreement with China, will require a localized digital strategy to build brand authority and disseminate scientific information in accordance with regional regulations and languages.
Moderna's website and digital presence effectively cover the core topic of mRNA science. The content explains the technology's mechanism, potential, and speed. It also provides detailed information on its key therapeutic areas: respiratory viruses, oncology, rare diseases, and latent viruses. However, there is an opportunity to expand topic coverage to address the implications of these technologies for patient outcomes and healthcare systems, moving from a purely scientific explanation to a value-based narrative that resonates with payers, providers, and patient advocacy groups.
Strategic Content Positioning
Moderna's content primarily serves the 'Awareness' and 'Consideration' stages for its diverse audiences. For investors, content like earnings reports and pipeline updates are crucial. For potential employees, the 'Life at Moderna' and employee stories (like Marta's) build an employer brand. For HCPs, the scientific publications and pipeline information serve as key resources. However, there is less content visible that supports the 'Decision' and 'Adoption' phase for HCPs, such as comparative efficacy data, health-economic outcomes, or tools for physician-patient conversations, which are critical for commercial success against competitors.
The primary thought leadership opportunity is to own the narrative around the future of mRNA beyond COVID-19. This involves creating content that frames mRNA as a versatile 'platform' technology capable of addressing humanity's most challenging diseases. While the website explains 'what is mRNA', it could more forcefully articulate a vision for personalized medicine, such as the individualized cancer vaccine (mRNA-4157). Showcasing the application of AI and other advanced technologies in their R&D process, as highlighted in the blog, can position Moderna at the intersection of biotech and tech, appealing to innovators and investors.
Competitors like Pfizer have extensive content ecosystems targeting HCPs with clinical resources, continuing medical education (CME), and patient support materials. Moderna's public-facing digital presence is less developed in these areas. There is a significant opportunity to create dedicated content hubs for specific therapeutic areas (e.g., an 'Oncology Hub') that provide deep, non-promotional scientific content, expert interviews, and clinical trial updates. This would build credibility and create a valuable resource for the medical community, helping to close the gap with more established pharmaceutical companies.
Moderna's core brand message—delivering on the promise of mRNA science to create transformative medicines—is clear and consistent across its homepage, 'about us' section, and employee stories. The visual identity and scientific, forward-looking tone are well-maintained. The tagline 'This Changes Everything' reflects its ambition. The challenge is ensuring this high-level corporate message is effectively translated into specific, value-driven messages for each product and therapeutic area as the pipeline matures.
Digital Market Strategy
Market Expansion Opportunities
- •
Develop dedicated, in-depth content hubs for each key pipeline area (Oncology, Rare Diseases, CMV) to establish authority and visibility in these new markets before product launches.
- •
Create localized content strategies for key international markets to support global expansion and partnership efforts.
- •
Target patient advocacy groups and communities with educational content around the diseases in their pipeline, building awareness and support at the grassroots level.
Customer Acquisition Optimization
- •
Invest in organic search visibility for non-branded, disease-specific keywords (e.g., 'CMV vaccine clinical trials', 'personalized oncology vaccines') to capture interest from HCPs and researchers, reducing reliance on paid channels.
- •
Develop a robust content marketing program for HCPs, including webinars, case studies, and expert panels, distributed through platforms like Doximity and LinkedIn to build relationships and generate qualified leads for medical science liaisons.
- •
Leverage employee stories and technology showcases (like the AI blog post) to attract top-tier talent, thereby optimizing recruitment marketing spend and improving the quality of applicants.
Brand Authority Initiatives
- •
Launch a thought leadership platform (e.g., a digital journal or podcast series) featuring Moderna's scientists and leaders discussing the future of medicine, AI in drug development, and the potential of the mRNA platform.
- •
Proactively create and distribute content that addresses misinformation about mRNA technology, reinforcing the science and safety data to build public and professional trust.
- •
Partner with leading medical institutions and key opinion leaders on co-created content to lend third-party credibility to Moderna's pipeline and technology.
Competitive Positioning Improvements
- •
Shift digital messaging from being a 'vaccine company' to a diversified 'mRNA therapeutics platform company' to differentiate from competitors and align with the long-term business strategy.
- •
Develop and publish health-economic outcome research and data visualizations that clearly articulate the value proposition of new therapies compared to existing standards of care, directly addressing the needs of payers and health systems.
- •
Highlight the speed and adaptability of the mRNA platform as a key competitive advantage in all corporate communications, emphasizing the ability to respond to future health crises and rapidly develop new treatments.
Business Impact Assessment
Success will be measured by the digital 'share of voice' for keywords related to pipeline products (e.g., CMV, personalized cancer vaccines) against competitors. Tracking lead volume and engagement from HCPs within new therapeutic areas via the digital platform will be a key indicator of future commercial traction.
Key metrics include the growth of an engaged HCP database through content subscriptions, webinar attendance, and resource downloads. For talent acquisition, success will be measured by the volume and quality of inbound applications from top-tier scientific and medical talent sourced through digital channels. For investors, metrics revolve around engagement with investor relations content and sentiment analysis in financial media.
Brand authority can be measured by an increase in unbranded organic search traffic, growth in citations of Moderna's scientific content in medical literature, media mentions that frame the company as a leader beyond COVID-19, and invitations for its leaders to speak at major industry and technology conferences.
Benchmarking will involve comparing Moderna's search engine visibility and share of voice for key strategic topics against primary competitors like Pfizer, BioNTech, and GSK. Success is defined by closing the visibility gap in established markets (like RSV) and establishing a leadership position in emerging markets (like mRNA-based oncology treatments).
Strategic Recommendations
High Impact Initiatives
- Initiative:
Launch 'The mRNA Future' Thought Leadership Hub
Business Impact:High
Market Opportunity:Solidify Moderna's position as the definitive leader in mRNA science, shaping the narrative for its entire pipeline and attracting investors, partners, and talent.
Success Metrics
- •
Media mentions citing the platform
- •
Growth in organic traffic for 'mRNA' related terms
- •
Inbound partnership inquiries
- •
Social media share of voice
- Initiative:
Develop a Targeted HCP Engagement Program for the Oncology Pipeline
Business Impact:High
Market Opportunity:Pre-condition the market for the launch of its personalized cancer vaccine by building early awareness, trust, and understanding among the oncology community.
Success Metrics
- •
Number of oncologists engaged (webinar attendees, content downloads)
- •
Lead generation for Medical Science Liaisons
- •
Sentiment analysis in physician social networks
- •
Website traffic to the oncology section
- Initiative:
Create a Content-Driven Talent Acquisition Funnel
Business Impact:Medium
Market Opportunity:Compete for scarce, elite scientific and AI talent by showcasing Moderna's innovative culture and cutting-edge work, reducing recruitment costs and time-to-hire.
Success Metrics
- •
Cost-per-hire for key scientific roles
- •
Quality of applicant score
- •
Engagement rate with 'Careers' and 'Culture' content
- •
Glassdoor rating improvements
The overarching strategy should be to deliberately evolve Moderna's market position from 'the COVID-19 vaccine company' to 'the world's leading mRNA therapeutics platform'. This requires a digital presence that consistently emphasizes the breadth of the pipeline, the underlying power of the technology platform, and the vision for treating a wide array of diseases. Every piece of content should reinforce the narrative of a diversified, next-generation biotechnology leader poised for long-term, multi-product growth.
Competitive Advantage Opportunities
- •
Leverage the company's authentic, tech-forward culture (e.g., adoption of AI) as a key differentiator to attract innovative talent and partners that larger, more traditional pharma companies cannot match.
- •
Emphasize the programmable and rapid-response nature of the mRNA platform as a durable competitive advantage, positioning Moderna as uniquely capable of addressing future health challenges.
- •
Build a direct communication channel with the medical and scientific community through high-value, non-promotional content, fostering a level of trust and transparency that builds a moat against competitors' marketing spend.
Moderna has successfully leveraged its pioneering role in the COVID-19 pandemic to build a globally recognized brand synonymous with mRNA innovation. Its current digital presence effectively communicates its scientific foundation, corporate mission, and ambitious pipeline. However, the company is at a critical strategic inflection point. Its market perception and digital visibility are still overwhelmingly tied to a single product, while its future success depends on commercializing a diverse portfolio in highly competitive therapeutic areas.
The primary strategic challenge is to transition its brand authority and market visibility from its past success into its future opportunities. Competitors like Pfizer and GSK possess deeply entrenched commercial relationships and extensive digital ecosystems for HCP engagement. Moderna's digital strategy must pivot from broad public awareness to targeted, high-value engagement with specific professional audiences: healthcare providers in oncology and infectious disease, potential research partners, and elite scientific talent.
The website content, such as the employee spotlight on using AI, provides a glimpse into a key competitive differentiator: a nimble, tech-forward culture. This must be amplified. The core strategic recommendation is to invest heavily in building out distinct, authoritative content hubs for each major pipeline area. These hubs should serve as the center of gravity for all digital marketing efforts, designed to establish credibility, educate the market, and build relationships with key opinion leaders and medical professionals long before product approvals. By focusing on demonstrating expertise and providing value to these niche audiences, Moderna can build a defensible market position that is less susceptible to the larger marketing budgets of its established competitors, ensuring its digital presence becomes a powerful engine for long-term growth and market leadership.
Strategic Priorities
Strategic Priorities
- Title:
Accelerate Commercialization of Flagship Pipeline Assets
Business Rationale:The company's over-reliance on a single, declining revenue stream (Spikevax) presents an existential risk. Successfully launching next-generation products in competitive markets like RSV and combination respiratory vaccines is critical to de-risk the business and demonstrate the platform's long-term commercial viability.
Strategic Impact:This initiative transforms Moderna from a single-product company into a sustainable, multi-product biopharmaceutical enterprise. It establishes new, durable revenue streams and validates the economic model of the entire mRNA platform, directly impacting long-term valuation and market leadership.
Success Metrics
- •
Revenue from non-COVID products exceeding 50% of total revenue by 2028
- •
Achieve #1 or #2 market share position for new respiratory vaccines within 24 months of launch
- •
Secure regulatory approval for at least two major non-COVID pipeline assets in the next 24 months
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Revenue Model
- Title:
Reposition Brand from 'COVID Vaccine Provider' to 'Diversified mRNA Platform Leader'
Business Rationale:Current market and investor perception is narrowly focused on past success, undervaluing the extensive and diverse pipeline. A deliberate brand shift is necessary to align perception with the company's long-term strategy and unlock the full valuation potential of its platform.
Strategic Impact:A successful repositioning will build a powerful brand narrative that supports market entry into new therapeutic areas like oncology and rare diseases. It will attract long-term investors, top-tier scientific talent, and strategic partners who understand the broader vision.
Success Metrics
- •
Increase in institutional investor ownership with an explicit biotech platform thesis
- •
Measurable shift in media share-of-voice from 'COVID' to 'oncology', 'rare disease', and 'mRNA platform'
- •
Improved recruitment metrics for senior scientific roles in non-vaccine therapeutic areas
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Brand Strategy
- Title:
Establish Strategic Alliances for Oncology Market Entry
Business Rationale:Entering the hyper-competitive oncology market requires immense commercial infrastructure, established physician relationships, and payer networks that Moderna currently lacks. Partnering with an established leader like Merck is the most capital-efficient and lowest-risk strategy to maximize the commercial potential of breakthrough assets like the personalized cancer vaccine (PCV).
Strategic Impact:This partnership strategy allows Moderna to penetrate a high-value market without a decade of prohibitive investment in building a commercial footprint from scratch. It accelerates time-to-revenue for the oncology pipeline and allows the company to focus on its core competency: R&D and platform innovation.
Success Metrics
- •
Successful execution of the co-commercialization agreement for mRNA-4157 (PCV)
- •
Achievement of pre-defined launch and sales milestones triggering partner payments
- •
Establishment of a repeatable partnership model for entering other complex therapeutic areas
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Partnerships
- Title:
Build a World-Class Commercial Engine for a Multi-Payer Market
Business Rationale:The go-to-market model for pandemic vaccines (large government contracts) is obsolete for the future portfolio. The company must build a sophisticated commercial organization adept at navigating complex reimbursement, pricing, and market access negotiations with a wide array of global payers.
Strategic Impact:This operational transformation is fundamental to converting scientific breakthroughs into profitable products. A robust commercial engine ensures that new therapies can gain broad market access, achieve favorable pricing, and effectively compete against entrenched pharmaceutical giants.
Success Metrics
- •
Average time from regulatory approval to securing broad formulary access in key markets
- •
Net price realization for new products relative to internal targets and competitors
- •
Recruitment and retention of a senior commercial leadership team with a proven track record in oncology and specialty pharma
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Operations
- Title:
Deploy Capital for Strategic Technology and Asset Acquisition
Business Rationale:Moderna's substantial cash reserve is a finite strategic asset that must be used to secure long-term competitive advantages. Acquiring complementary technologies (e.g., novel delivery systems beyond LNPs) or late-stage clinical assets will widen the company's technological moat and accelerate diversification.
Strategic Impact:Strategic capital deployment transforms cash on the balance sheet into durable competitive advantages. It can solve key technical challenges (like tissue-specific delivery), reduce reliance on the success of the internal pipeline, and accelerate the company's expansion into new therapeutic modalities.
Success Metrics
- •
Completion of 1-2 strategic acquisitions in core technology areas (e.g., delivery, AI) within 18 months
- •
Integration of acquired technology leading to the initiation of a new pipeline program
- •
Increase in the number of therapeutic areas addressable by the company's platform
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Operations
Moderna must rapidly pivot from its single-product, pandemic-era identity into a diversified biopharmaceutical leader. This requires flawlessly executing on its late-stage pipeline to create new revenue streams while strategically repositioning its brand to reflect the broad, revolutionary potential of its core mRNA platform.
The company's core competitive advantage is its pioneering, digitally-integrated mRNA platform, which enables unprecedented speed and programmability in designing and developing novel medicines, from next-generation vaccines to personalized cancer therapies.
The primary growth catalyst will be the successful commercialization of one or more blockbuster therapies from its late-stage pipeline, particularly in combination respiratory vaccines or personalized oncology. This will definitively prove the platform's value beyond COVID-19 and unlock durable, long-term revenue growth.