eScore
pfizer.comThe eScore is a comprehensive evaluation of a business's online presence and effectiveness. It analyzes multiple factors including digital presence, brand communication, conversion optimization, and competitive advantage.
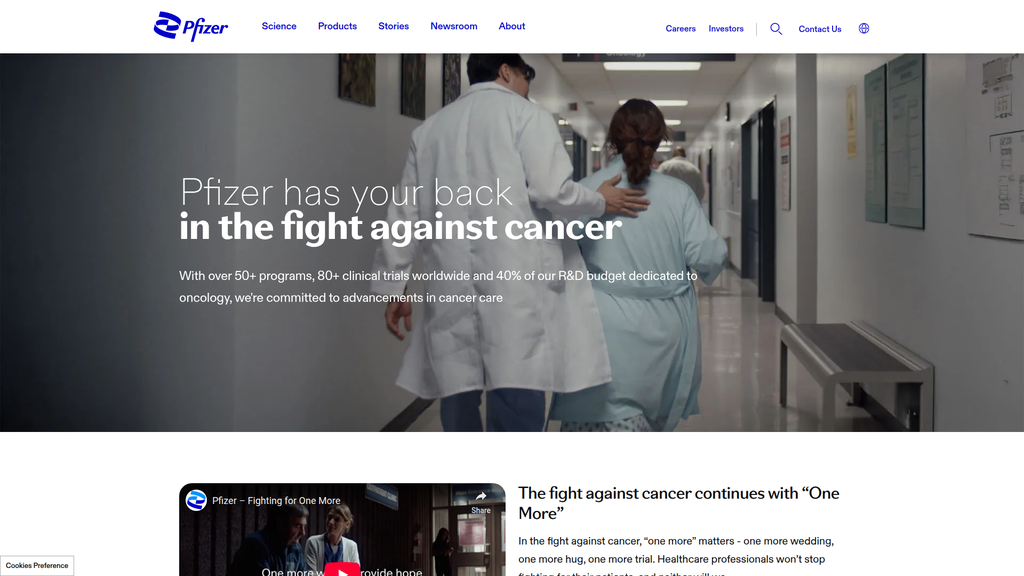
Pfizer maintains a world-class digital presence, demonstrating high content authority and a strong, consistent multi-channel strategy. The website's information architecture is robust, aligning well with the search intent of diverse audiences like patients, healthcare professionals (HCPs), and investors. Its global reach is extensive, though the primary .com site is geared towards the U.S., indicating opportunities for deeper localization.
Exceptional content authority, reinforced by transparency initiatives like the 'Funded Initiatives' page, which attracts high-quality backlinks from academic and medical institutions.
Enhance geographic market penetration by developing more dedicated, localized content hubs for key international markets beyond the primary English-language site.
Pfizer's messaging is powerful and effective, particularly its emotive, patient-centric campaign around oncology, which strongly resonates with its target audience. The brand successfully segments its messaging for different personas, from empathetic narratives for patients to data-heavy content for investors and HCPs. However, the intense focus on oncology on the homepage risks overshadowing other key therapeutic areas like vaccines and immunology.
Masterful use of emotional storytelling in its oncology messaging ('one more hug, one more wedding') to build a strong, empathetic connection with patients and caregivers.
Revise the homepage message hierarchy to create a more balanced representation of all core therapeutic areas, preventing the over-focus on oncology from diminishing the perceived importance of other major divisions.
The website provides a clear, low-friction experience for its primary goal: information dissemination to key audiences. Navigation is intuitive, and the cognitive load is generally light, guiding users to relevant content hubs effectively. While traditional e-commerce conversions aren't the goal, micro-conversions like newsletter sign-ups or accessing patient support could be improved with more prominent, action-oriented CTAs.
An intuitive and logical information architecture that allows diverse audiences (patients, HCPs, investors) to self-segment and find relevant information with minimal friction.
Conduct A/B testing on key Calls-to-Action (CTAs) to replace passive language like 'Learn More' with more compelling, action-oriented copy such as 'Discover Our Pipeline' to improve engagement.
Credibility is exceptionally high, built on a foundation of scientific authority, a 175-year history, and robust third-party validation through extensive partnerships. The website features a strong hierarchy of trust signals, including detailed pipeline data and compliance with transparency laws like the Sunshine Act. The company's commitment to data privacy and security is well-documented, mitigating risk for users.
Unparalleled transparency through the 'Funded Initiatives' page, which publicly discloses financial relationships with healthcare professionals, building significant trust and demonstrating compliance.
Publish a formal, easily accessible 'Accessibility Statement' detailing commitment to WCAG 2.1 AA standards to solidify its compliance posture and mitigate legal risk under the ADA.
Pfizer's competitive advantage is deeply entrenched and sustainable, rooted in its massive scale of R&D, global commercial infrastructure, and extensive patent portfolio. The recent acquisition of Seagen has fortified its moat in oncology, providing a proprietary technology platform (ADCs) that is difficult for competitors to replicate. While it faces the constant threat of patent cliffs, its innovation engine is designed to consistently replenish its product portfolio.
The sheer scale of its integrated R&D, manufacturing, and global commercialization network provides a durable competitive advantage that is nearly impossible for new entrants to replicate.
Address the high revenue concentration on a few blockbuster drugs by continuing to aggressively pursue pipeline diversification and successful 'bolt-on' acquisitions to de-risk future patent expirations.
The business model is inherently scalable due to high operating leverage, where high fixed R&D costs are offset by high-margin sales of successful drugs. Pfizer has mature processes for global market expansion and a strong track record of entering new therapeutic areas via M&A. Future scalability is tied to R&D productivity and successfully navigating global regulatory hurdles for its 100+ program pipeline.
A highly efficient capital allocation strategy that balances returning capital to shareholders with aggressive reinvestment in both internal R&D and strategic, large-scale acquisitions to fuel future growth.
Increase investment in internal AI and machine learning capabilities to accelerate drug discovery and shorten clinical trial timelines, reducing a key operational bottleneck for future growth.
Pfizer's business model demonstrates exceptional coherence, with a clear strategic pivot to dominate the high-growth oncology market. The $43B acquisition of Seagen is a decisive resource allocation move that directly supports this strategic focus, aligning its activities, partnerships, and cost structure with its primary goal. This sharpened focus on science-driven breakthroughs ensures strong alignment among stakeholders toward a clear, unified vision for future growth.
A clear and decisive strategic focus on oncology, demonstrated by the massive capital allocation for the Seagen acquisition, which aligns the entire organization towards a single, high-growth objective.
Proactively develop and pilot value-based pricing models with payers to better align the revenue model with the growing global pressure for outcome-based healthcare, mitigating future regulatory risk.
As one of the world's largest pharmaceutical companies, Pfizer wields immense market power, consistently maintaining a top-tier market share and demonstrating significant pricing power for its innovative, patent-protected medicines. Its ability to execute mega-deals like the Seagen acquisition, shape treatment guidelines through extensive clinical data, and influence the direction of medical research gives it substantial market influence. Its global scale also provides considerable leverage with suppliers and partners.
Dominant market position and brand equity, which provide significant pricing power for novel therapies and strong leverage in negotiating with payers, partners, and suppliers globally.
Mitigate customer dependency risk by ensuring the successful launch of multiple new products from the pipeline to offset the ~$17 billion revenue loss expected from patent expirations between 2025-2030.
Business Overview
Business Classification
Biopharmaceutical R&D and Commercialization
Biologics and Advanced Therapies Development
Pharmaceuticals
Sub Verticals
- •
Oncology
- •
Vaccines
- •
Internal Medicine
- •
Inflammation & Immunology
- •
Rare Disease
Mature
Maturity Indicators
- •
Extensive global commercial infrastructure
- •
Long history of operations (founded 1849)
- •
Consistent dividend payments for over 50 years.
- •
Large-scale M&A activity, including the $43B acquisition of Seagen.
- •
Deep and diverse R&D pipeline with over 100 programs.
- •
Significant brand equity and established market presence
Enterprise
Steady
Revenue Model
Primary Revenue Streams
- Stream Name:
Innovative Medicines & Vaccines (Patented)
Description:Sales of branded, patent-protected prescription drugs and vaccines across key therapeutic areas. Key products include the Prevnar family, Eliquis, Vyndaqel, and oncology drugs like Ibrance and Padcev.
Estimated Importance:Primary
Customer Segment:Healthcare Providers, Payers, Governments
Estimated Margin:High
- Stream Name:
Oncology Portfolio
Description:A strategic growth pillar, significantly enhanced by the Seagen acquisition, focusing on antibody-drug conjugates (ADCs), small molecules, and bispecific antibodies. The goal is to have at least eight blockbuster cancer drugs by 2030.
Estimated Importance:Primary
Customer Segment:Oncologists, Specialized Cancer Centers, Payers
Estimated Margin:High
- Stream Name:
COVID-19 Franchise (Comirnaty & Paxlovid)
Description:Revenue from the COVID-19 vaccine (Comirnaty) and oral antiviral treatment (Paxlovid). While declining from pandemic peaks, it remains a significant contributor.
Estimated Importance:Secondary
Customer Segment:Governments, Healthcare Systems, Pharmacies
Estimated Margin:Medium
- Stream Name:
Established Medicines & Biosimilars
Description:Sales of off-patent and generic drugs where Pfizer retains brand recognition or manufacturing scale, as well as a growing portfolio of biosimilars.
Estimated Importance:Tertiary
Customer Segment:Pharmacies, Healthcare Providers
Estimated Margin:Low
Recurring Revenue Components
- •
Therapies for chronic conditions (e.g., cardiovascular, immunology)
- •
Long-term government vaccine contracts
- •
Multi-year treatment regimens for oncology
Pricing Strategy
Value-Based & Negotiated Pricing
Premium
Opaque
Pricing Psychology
- •
Justification through clinical superiority data
- •
Value-based arguments to payers
- •
Patient assistance programs to mitigate out-of-pocket costs
Monetization Assessment
Strengths
- •
High margins on patented, innovative products
- •
Diverse portfolio mitigates risk from any single product failure
- •
Strong negotiating power with payers due to market leadership and critical therapies
Weaknesses
- •
Significant revenue loss from patent expirations ('patent cliff')
- •
High and risky R&D investments required to sustain the pipeline
- •
Dependence on a few blockbuster drugs for a large portion of revenue
Opportunities
- •
Expansion of oncology portfolio, leveraging Seagen's ADC technology, to drive future growth.
- •
Growth in biologics and next-generation therapies like mRNA and gene therapy
- •
Penetration into emerging markets with growing healthcare spending
Threats
- •
Increasing pricing pressure from governments and insurers, such as the US Inflation Reduction Act (IRA).
- •
Competition from generic and biosimilar manufacturers post-patent expiry
- •
R&D pipeline failures or delays in regulatory approvals
- •
Increased competition from agile biotech firms and other large pharma companies.
Market Positioning
Science-Driven Innovation Leader
Top-Tier Global Pharmaceutical Company
Target Segments
- Segment Name:
Healthcare Providers (HCPs)
Description:Physicians, specialists (e.g., oncologists, cardiologists), nurses, and hospital systems who prescribe and administer Pfizer's products.
Demographic Factors
Licensed medical professionals
Specialization in therapeutic areas like oncology, immunology, etc.
Psychographic Factors
- •
Driven by clinical data and evidence-based medicine
- •
Seeking best possible outcomes for patients
- •
Value safety, efficacy, and brand reputation
Behavioral Factors
- •
Attend medical conferences
- •
Read peer-reviewed journals
- •
Influenced by Key Opinion Leaders (KOLs) and medical science liaisons
Pain Points
- •
Limited treatment options for complex diseases
- •
Navigating complex reimbursement and payer requirements
- •
Keeping up-to-date with the latest clinical advancements
Fit Assessment:Excellent
Segment Potential:High
- Segment Name:
Payers & Health Systems
Description:Public and private insurance companies, pharmacy benefit managers (PBMs), and national health systems that manage drug formularies and reimburse costs.
Demographic Factors
Large organizations managing significant healthcare budgets
Psychographic Factors
Focus on cost-effectiveness and budget impact
Value long-term health outcomes and total cost of care reduction
Behavioral Factors
Conduct health technology assessments (HTAs)
Negotiate rebates and pricing based on volume and clinical value
Pain Points
Rising cost of specialty drugs
Managing budgets while ensuring patient access to innovative medicines
Fit Assessment:Good
Segment Potential:High
- Segment Name:
Patients & Caregivers
Description:Individuals with acute or chronic diseases who are the end-users of Pfizer's medicines, and their support networks.
Demographic Factors
Varies widely by disease state (age, gender, etc.)
Psychographic Factors
- •
Seeking hope, improved quality of life, and longevity
- •
Value trust, transparency, and support
- •
Concerned about affordability and side effects
Behavioral Factors
- •
Increasingly research conditions and treatments online
- •
Participate in patient advocacy groups
- •
Respond to Direct-to-Consumer (DTC) advertising
Pain Points
- •
High out-of-pocket costs
- •
Accessing and navigating complex healthcare systems
- •
Managing the physical and emotional burden of illness
Fit Assessment:Excellent
Segment Potential:High
Market Differentiation
- Factor:
R&D Scale and Pipeline Depth
Strength:Strong
Sustainability:Sustainable
- Factor:
Global Commercialization & Manufacturing Infrastructure
Strength:Strong
Sustainability:Sustainable
- Factor:
Leadership in Key Technology Platforms (e.g., mRNA, ADCs)
Strength:Strong
Sustainability:Sustainable
- Factor:
Brand Equity and Long-Standing Reputation
Strength:Moderate
Sustainability:Sustainable
Value Proposition
To deliver breakthroughs that change patients' lives through pioneering science, a world-class R&D engine, and global commercial reach.
Excellent
Key Benefits
- Benefit:
Novel therapies for unmet medical needs
Importance:Critical
Differentiation:Unique
Proof Elements
- •
Extensive R&D pipeline data
- •
FDA/EMA approvals for new molecular entities
- •
Acquisition of innovative companies like Seagen.
- Benefit:
Clinically proven safety and efficacy
Importance:Critical
Differentiation:Common
Proof Elements
- •
Rigorous multi-phase clinical trial data
- •
Peer-reviewed publications in top medical journals
- •
Regulatory approvals from global health authorities
- Benefit:
Patient access and affordability support
Importance:Important
Differentiation:Somewhat unique
Proof Elements
- •
Pfizer RxPathways program
- •
Co-pay assistance programs
- •
Partnerships with patient advocacy groups
Unique Selling Points
- Usp:
End-to-end integration from discovery to global commercialization
Sustainability:Long-term
Defensibility:Strong
- Usp:
Proprietary Antibody-Drug Conjugate (ADC) technology platform via Seagen
Sustainability:Long-term
Defensibility:Strong
- Usp:
Proven leadership and manufacturing scale in advanced modalities like mRNA vaccines
Sustainability:Medium-term
Defensibility:Moderate
Customer Problems Solved
- Problem:
Life-threatening diseases with limited or no treatment options (e.g., specific cancers)
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Chronic conditions that impair quality of life (e.g., inflammatory diseases)
Severity:Major
Solution Effectiveness:Partial
- Problem:
Preventable infectious diseases (e.g., pneumonia, COVID-19)
Severity:Critical
Solution Effectiveness:Complete
Value Alignment Assessment
High
Pfizer's focus on high-burden diseases like cancer, cardiovascular conditions, and infectious diseases directly aligns with the most significant unmet needs in global public health.
High
The value proposition of providing effective, life-altering medicines resonates powerfully with patients, prescribers, and health systems alike, addressing their core needs for better health outcomes.
Strategic Assessment
Business Model Canvas
Key Partners
- •
Biotechnology companies (e.g., BioNTech, 3SBio Inc. )
- •
Academic and research institutions
- •
Contract Research Organizations (CROs)
- •
Governments and public health organizations
- •
Other pharmaceutical companies (for co-promotion/development)
Key Activities
- •
Research & Development (Drug Discovery, Clinical Trials)
- •
Manufacturing and Supply Chain Management
- •
Regulatory Affairs and Compliance
- •
Sales, Marketing, and Market Access
- •
Mergers & Acquisitions / Business Development.
Key Resources
- •
Intellectual Property (Patents)
- •
Scientific and technical talent
- •
Global manufacturing and distribution networks
- •
Strong balance sheet and access to capital
- •
Brand reputation and established relationships with stakeholders
Cost Structure
- •
Research & Development (R&D) expenses.
- •
Sales, General & Administrative (SG&A) expenses
- •
Cost of Goods Sold (COGS)
- •
M&A and in-licensing deal costs
Swot Analysis
Strengths
- •
Diversified, high-margin product portfolio.
- •
Industry-leading R&D budget and pipeline.
- •
Unmatched global scale in manufacturing and commercialization
- •
Strong financial position and cash flow
- •
Strategic pivot to oncology with Seagen acquisition doubles pipeline and adds ADC leadership.
Weaknesses
- •
High vulnerability to patent expirations for key blockbusters (e.g., Eliquis, Ibrance).
- •
Recent clinical trial setbacks in some areas.
- •
Post-pandemic revenue decline from COVID-19 products creates growth pressure.
Opportunities
- •
Become a dominant leader in oncology, projected to be the largest growth driver in global medicine.
- •
Leverage AI and machine learning to accelerate drug discovery and reduce R&D costs.
- •
Expand into new high-growth therapeutic areas like obesity and rare diseases
- •
Further growth in emerging markets
Threats
- •
Intensifying government price controls and rebate pressures (e.g., US Inflation Reduction Act).
- •
Increasing cost and complexity of bringing new drugs to market
- •
Heightened competition from both large pharma rivals and innovative biotech firms.
- •
Potential for major litigation and regulatory scrutiny
Recommendations
Priority Improvements
- Area:
Oncology Integration & Execution
Recommendation:Aggressively execute on the Seagen integration to accelerate the development of its ADC pipeline and achieve the goal of 8+ oncology blockbusters by 2030. Ensure seamless commercial integration to maximize the value of acquired assets.
Expected Impact:High
- Area:
R&D Productivity and Pipeline Prioritization
Recommendation:Continue disciplined R&D spending by focusing investments on high-potential modalities (ADCs, biologics) and therapeutic areas. Utilize AI and real-world evidence to improve clinical trial success rates and shorten timelines.
Expected Impact:High
- Area:
Navigating the Post-Patent Cliff Environment
Recommendation:Develop and execute a clear lifecycle management and business development strategy to offset the ~$17B in revenue expected to be lost to patent expirations between 2025-2030.
Expected Impact:High
Business Model Innovation
- •
Develop 'Beyond the Pill' integrated solutions, combining therapeutics with digital health tools, diagnostics, and patient support services to create a comprehensive care ecosystem, particularly in oncology.
- •
Expand outcome-based and value-based pricing agreements with payers, tying reimbursement directly to the clinical effectiveness of high-cost therapies.
- •
Establish a more agile, semi-autonomous biotech-like R&D unit focused on high-risk, high-reward science to foster faster innovation, separate from the core commercial engine.
Revenue Diversification
- •
Strategically invest in or acquire companion diagnostics companies to create integrated treatment paradigms, ensuring the right patients are identified for Pfizer's targeted therapies.
- •
Build out a dedicated Contract Development and Manufacturing Organization (CDMO) service to leverage Pfizer's world-class manufacturing capacity, especially for complex biologics and ADCs.
- •
Explore ethical monetization of anonymized real-world data and clinical trial data to provide insights to the broader healthcare community, creating a new high-margin revenue stream.
Pfizer is a mature, top-tier biopharmaceutical enterprise at a critical strategic inflection point. Its business model, traditionally reliant on developing and commercializing a diverse portfolio of blockbuster small-molecule drugs and vaccines, is evolving to navigate the immense pressure of impending patent cliffs and a changing regulatory landscape. The company's future growth trajectory is now unequivocally centered on becoming a dominant force in oncology. The $43 billion acquisition of Seagen is the cornerstone of this strategic pivot, transforming Pfizer's pipeline and positioning it as a leader in the highly promising field of Antibody-Drug Conjugates (ADCs). This move is a clear effort to shift its revenue base towards more durable, higher-margin biologics, which are less susceptible to traditional generic competition. While the post-pandemic decline in COVID-19 product revenues has created short-term growth headwinds, the company's core business remains robust, and its deep pipeline of over 100 programs provides multiple opportunities for future blockbusters. The primary challenge for Pfizer's business model is execution: it must seamlessly integrate Seagen, accelerate its oncology pipeline, and effectively manage the significant loss of exclusivity for key products. Success will depend on enhancing R&D productivity, maintaining disciplined cost management, and strategically deploying capital to further bolster its innovation engine. The evolution from a diversified pharmaceutical giant to a more focused, science-driven oncology leader is the central narrative that will define its market position and financial performance through 2030.
Competitors
Competitive Landscape
Mature
Oligopoly
Barriers To Entry
- Barrier:
High Research & Development (R&D) Costs
Impact:High
- Barrier:
Stringent Regulatory Approval (e.g., FDA, EMA)
Impact:High
- Barrier:
Intellectual Property (Patents)
Impact:High
- Barrier:
Complex Manufacturing and Supply Chains
Impact:High
- Barrier:
Established Distribution Networks and Market Access
Impact:Medium
Industry Trends
- Trend:
Rise of Artificial Intelligence in Drug Discovery
Impact On Business:Potential to significantly shorten R&D timelines and reduce costs, creating opportunities for nimbler, tech-focused competitors.
Timeline:Immediate
- Trend:
Personalized Medicine and Cell/Gene Therapies
Impact On Business:Shifts focus from blockbuster drugs for large populations to highly targeted, high-value treatments, requiring new manufacturing and commercialization models.
Timeline:Near-term
- Trend:
Patent Cliff and Growth of Biosimilars
Impact On Business:Increasing revenue erosion from key products as they lose exclusivity, necessitating a robust and innovative R&D pipeline to compensate.
Timeline:Immediate
- Trend:
Increased Pricing Pressure and Regulatory Scrutiny
Impact On Business:Margin pressure from governments and payers demanding more value, affecting profitability and forcing greater operational efficiency.
Timeline:Immediate
- Trend:
Digital Transformation and Patient-Centricity
Impact On Business:Competition is shifting from 'Share-of-Voice' with physicians to engaging patients directly through digital channels and data-driven services.
Timeline:Near-term
Direct Competitors
- →
Johnson & Johnson (Innovative Medicine)
Market Share Estimate:High
Target Audience Overlap:High
Competitive Positioning:Diversified global leader in pharmaceuticals, medical devices, and consumer health, emphasizing broad-spectrum innovation.
Strengths
- •
Extremely diversified portfolio across multiple healthcare segments.
- •
Strong global brand recognition and trust.
- •
Dominant positions in immunology (e.g., Stelara) and oncology.
- •
Robust supply chain and global distribution network.
Weaknesses
- •
Facing significant patent expirations on key blockbuster drugs like Stelara.
- •
Large size can lead to slower adaptation to new technologies compared to smaller biotechs.
- •
Ongoing litigation risks in various business segments.
Differentiators
- •
Synergies between pharmaceutical, MedTech, and consumer health divisions.
- •
Aggressive M&A strategy to acquire innovation.
- •
Long-standing history and established relationships with healthcare systems worldwide.
- →
Merck & Co., Inc.
Market Share Estimate:High
Target Audience Overlap:High
Competitive Positioning:Research-intensive biopharmaceutical company with a strong focus on oncology and vaccines.
Strengths
- •
Market leadership in immuno-oncology with blockbuster drug Keytruda.
- •
Strong portfolio of vaccines (e.g., Gardasil).
- •
Deep R&D expertise and a history of scientific breakthroughs.
- •
Efficient and focused commercial organization.
Weaknesses
- •
High revenue concentration on Keytruda, creating significant risk around its eventual patent expiration.
- •
Less diversified therapeutic area focus compared to J&J or Roche.
- •
Facing increased competition in the oncology space.
Differentiators
- •
Pioneering position in immuno-oncology.
- •
Legacy of leadership in vaccine development.
- •
Strategic focus on developing foundational therapies that can be used across many cancer types.
- →
Novartis AG
Market Share Estimate:High
Target Audience Overlap:High
Competitive Positioning:A 'pure-play' innovative medicines company focused on high-growth therapeutic areas like cardiovascular, immunology, and oncology.
Strengths
- •
Strong portfolio of high-growth, in-market brands like Entresto and Cosentyx.
- •
Leadership in advanced therapy platforms like cell and gene therapy.
- •
Strategic shift to focus solely on innovative medicines after spinning off Sandoz (generics).
- •
Robust R&D pipeline with a stated goal for new medicines to have >$2 billion peak sales potential.
Weaknesses
- •
Faces a significant patent cliff starting around 2026.
- •
Increased risk profile by divesting the more stable generics business.
- •
Historically lagged some competitors in the immuno-oncology space.
Differentiators
- •
Clear strategic focus on innovative, high-value medicines.
- •
Strong presence in complex and cutting-edge technology platforms.
- •
Aggressive in portfolio transformation through M&A and divestitures.
- →
Roche Holding AG
Market Share Estimate:High
Target Audience Overlap:High
Competitive Positioning:Global leader in pharmaceuticals and diagnostics, pioneering personalized healthcare.
Strengths
- •
Unmatched leadership in oncology and in-vitro diagnostics.
- •
Synergy between pharmaceutical and diagnostics divisions enables a strong personalized medicine strategy.
- •
Extensive portfolio of blockbuster biologic drugs (e.g., Herceptin, Avastin), though many now face biosimilar competition.
- •
Considered a leader in AI implementation for drug discovery and diagnostics.
Weaknesses
- •
Significant revenue loss due to biosimilar competition for its older oncology drugs.
- •
Pipeline has faced some high-profile setbacks in recent years.
- •
Competition is intensifying in its core areas of oncology and neurology.
Differentiators
- •
Integrated 'Pharma + Diagnostics' business model is a core strategic advantage.
- •
Decades of dominance and data in oncology.
- •
Heavy investment in data science and genomics to drive future growth.
Indirect Competitors
- →
Biotech Companies (e.g., Moderna, BioNTech)
Description:Innovators focused on novel technology platforms like mRNA. They are often partners (as BioNTech is with Pfizer) but also represent a disruptive force, capable of developing new therapeutic classes independently.
Threat Level:Medium
Potential For Direct Competition:High, as they build out their own pipelines and commercial capabilities.
- →
Generic & Biosimilar Manufacturers (e.g., Sandoz, Teva, Amgen)
Description:These companies compete on price by marketing equivalent versions of originator drugs after patent expiry, directly eroding the revenue of Pfizer's established products.
Threat Level:High
Potential For Direct Competition:They do not compete with innovative/patented products, but are a direct competitor to off-patent assets.
- →
AI Drug Discovery Companies (e.g., Insilico Medicine, Exscientia)
Description:Technology companies using AI and machine learning to dramatically accelerate the identification and design of new drug candidates. While often partners, they are changing the R&D landscape and could eventually develop their own assets.
Threat Level:Low
Potential For Direct Competition:Medium in the long-term, as their platforms mature.
- →
Big Tech (e.g., Google/Verily, NVIDIA)
Description:Entering the healthcare space by leveraging massive datasets, computational power, and AI to transform clinical trials, diagnostics, and drug discovery, posing a long-term disruptive threat.
Threat Level:Low
Potential For Direct Competition:Low for developing and selling drugs, but high for disrupting the value chain.
Competitive Advantage Analysis
Sustainable Advantages
- Advantage:
Massive Scale of R&D Operations
Sustainability Assessment:High
Competitor Replication Difficulty:Hard
- Advantage:
Extensive Global Commercial Infrastructure and Distribution Network
Sustainability Assessment:High
Competitor Replication Difficulty:Hard
- Advantage:
Broad and Diversified Patent Portfolio
Sustainability Assessment:Medium to High
Competitor Replication Difficulty:Hard
- Advantage:
Strong Brand Recognition and Trust among Healthcare Professionals and Patients
Sustainability Assessment:High
Competitor Replication Difficulty:Medium
Temporary Advantages
{'advantage': 'Market Exclusivity on Blockbuster Drugs', 'estimated_duration': 'Varies by patent expiration dates (e.g., Eliquis, Ibrance).'}
{'advantage': 'First-Mover Advantage in a New Therapeutic Class (e.g., mRNA vaccines)', 'estimated_duration': '2-4 years before competitors launch similar products.'}
Disadvantages
- Disadvantage:
Dependence on a few blockbuster drugs for a significant portion of revenue.
Impact:Major
Addressability:Difficult
- Disadvantage:
Public and political pressure on drug pricing.
Impact:Major
Addressability:Difficult
- Disadvantage:
Large corporate structure can slow down innovation and adaptation compared to smaller, more agile biotechs.
Impact:Minor
Addressability:Moderately
Strategic Recommendations
Quick Wins
- Recommendation:
Enhance digital content for Healthcare Professionals (HCPs) by creating dedicated microsites for key products with easily accessible clinical data, trial results, and prescribing information, moving beyond general corporate messaging.
Expected Impact:Medium
Implementation Difficulty:Easy
- Recommendation:
Launch targeted social media campaigns highlighting patient stories and outcomes related to key therapeutic areas (like oncology, as emphasized on the homepage) to build brand sentiment and patient trust.
Expected Impact:Medium
Implementation Difficulty:Moderate
Medium Term Strategies
- Recommendation:
Aggressively pursue 'bolt-on' acquisitions of mid-stage biotech companies with promising assets in Pfizer's core therapeutic areas to de-risk the R&D pipeline and fill potential revenue gaps from patent expirations.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Establish a formal 'Digital Therapeutics' division to develop and partner on FDA-approved software and digital tools that complement Pfizer's existing drug portfolio, creating integrated treatment solutions.
Expected Impact:High
Implementation Difficulty:Moderate
- Recommendation:
Expand the 'Accord for a Healthier World' initiative into a broader educational platform for HCPs in emerging markets, building long-term brand equity and market access.
Expected Impact:Medium
Implementation Difficulty:Moderate
Long Term Strategies
- Recommendation:
Invest heavily in internal AI and machine learning capabilities to institutionalize accelerated drug discovery, aiming to reduce the average time from target identification to clinical trials.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Diversify into new, high-science therapeutic modalities (e.g., expanding beyond mRNA into areas like CRISPR-based therapies) to create the next wave of sustainable competitive advantages.
Expected Impact:High
Implementation Difficulty:Difficult
Solidify Pfizer's position as the leader in 'Science-Driven Breakthroughs for Patients.' This moves beyond a general pharma identity to a more focused narrative emphasizing cutting-edge R&D (pipeline size, investment) and its direct impact on patient lives (as shown in homepage messaging).
Differentiate through 'Integrated Patient Support.' While competitors focus on the drug, Pfizer can win by owning the patient journey. This means scaling programs like RxPathways and integrating digital health tools, patient education, and support communities to improve outcomes and build loyalty beyond the pill.
Whitespace Opportunities
- Opportunity:
Digital Health and Therapeutics Integration
Competitive Gap:Most large pharma companies have been slow to integrate regulated digital therapeutics (DTx) with their drug offerings. There is a gap in providing a combined 'drug + digital' package to improve adherence and outcomes.
Feasibility:Medium
Potential Impact:High
- Opportunity:
Building Branded Patient Communities
Competitive Gap:Competitor websites are largely corporate-facing. A significant opportunity exists to create secure, valuable, disease-specific online communities for patients, fostering direct engagement and gathering real-world evidence.
Feasibility:Medium
Potential Impact:Medium
- Opportunity:
Proactive Value-Based Care Partnerships
Competitive Gap:While many talk about value-based care, few have implemented large-scale partnerships with payers and health systems that tie drug reimbursement to patient outcomes. Leading in this area would be a powerful differentiator.
Feasibility:Low
Potential Impact:High
- Opportunity:
AI-Powered Clinical Trial Recruitment
Competitive Gap:All pharma companies struggle with slow and inefficient clinical trial recruitment. Investing in and scaling an AI platform to identify and enroll patients faster could become a significant operational advantage, speeding all products to market.
Feasibility:Medium
Potential Impact:High
Pfizer operates within a mature, oligopolistic biopharmaceutical industry characterized by intense competition, high barriers to entry, and transformative technological shifts. The primary competitive arena is a race of innovation, where companies battle to develop and commercialize novel, patent-protected drugs to offset inevitable revenue losses from patent expirations—the so-called 'patent cliff.'
Direct competition is fierce, with global giants like Johnson & Johnson, Merck, Novartis, and Roche vying for market share across key therapeutic areas, particularly oncology, immunology, and vaccines. Each competitor possesses unique strengths: Merck's dominance in immuno-oncology with Keytruda, Roche's integrated diagnostics and pharma model, and Novartis's focused innovation strategy. Pfizer's competitive strength lies in its immense scale, deep R&D pipeline (as highlighted on its website), and global commercial reach. However, like its peers, it faces the constant threat of revenue erosion from biosimilars and generic drugs.
The competitive landscape is being reshaped by powerful external trends. The rise of AI in drug discovery threatens to shorten R&D cycles, potentially leveling the playing field for smaller, tech-savvy players. Simultaneously, the move toward personalized medicine demands new capabilities in diagnostics and targeted therapies. Indirect competitors, from agile biotech firms to data-centric tech giants, are further disrupting the traditional value chain.
Pfizer's digital presence, as seen on its website, effectively communicates its core purpose of 'breakthroughs that change patients' lives,' with a strong emphasis on its oncology pipeline and patient support programs like RxPathways. This narrative is crucial in an era where competition is shifting from a pure 'share of voice' with physicians to a more patient-centric model. The key strategic challenge for Pfizer is to leverage its scale and R&D engine to consistently produce high-value innovations while becoming more agile to embrace new technologies and digital patient engagement models. Future success will be defined not just by the next blockbuster drug, but by the ability to build an integrated ecosystem of treatments, digital support, and data-driven insights to deliver superior patient outcomes.
Messaging
Message Architecture
Key Messages
- Message:
Pfizer has your back in the fight against cancer.
Prominence:Primary
Clarity Score:High
Location:Homepage Hero Section
- Message:
Breakthroughs that change patients' lives.
Prominence:Primary
Clarity Score:High
Location:Implied through multiple headings and company mission
- Message:
We're not waiting for breakthroughs - we're working to create them.
Prominence:Secondary
Clarity Score:High
Location:Homepage Content Section
- Message:
Powered by science and advanced technologies, we are committed to rapidly advancing novel combinations and next-generation biologics.
Prominence:Secondary
Clarity Score:Medium
Location:Homepage Content Section
- Message:
Where people live shouldn’t impact the quality of their healthcare and income shouldn’t determine health outcomes.
Prominence:Tertiary
Clarity Score:High
Location:Homepage 'An Accord for a Healthier World' Section
The message hierarchy on the homepage is exceptionally clear, with a dominant, primary focus on oncology. This is a deliberate strategic choice to position Pfizer as a leader in a high-profile, critical disease area. While effective, this strong focus risks overshadowing Pfizer's significant work in other key areas like vaccines, internal medicine, and immunology, which are relegated to secondary navigation elements.
Messaging is largely consistent in its core themes of scientific innovation and patient impact. The tone, however, shifts significantly from the emotive, patient-centric language on the homepage to a highly technical and formal tone on interior pages like 'Funded Initiatives'. This is appropriate for the different audiences but creates a somewhat disjointed brand experience.
Brand Voice
Voice Attributes
- Attribute:
Authoritative
Strength:Strong
Examples
Every product is the result of 1,500 scientists overseeing more than 500,000 lab tests and over 36 clinical trials before the first prescription.
Pipeline Snapshot as of August 5, 2025
- Attribute:
Hopeful & Empathetic
Strength:Strong
Examples
- •
In the fight against cancer, “one more” matters - one more wedding, one more hug, one more trial.
- •
We're committed to advancements in cancer care.
- •
So that people with cancer can live better and longer lives.
- Attribute:
Scientific & Technical
Strength:Strong
Examples
- •
Advancing Cancer Care with Cutting-Edge Science
- •
We look for treatments that provide more than just symptom relief, in order to address the root cause of chronic inflammatory diseases at a molecular level
- •
Pfizer will publish full proposals of funded initiatives...
- Attribute:
Corporate & Formal
Strength:Moderate
Examples
Pfizer's Global Health Fellows Program: Leveraging Colleagues’ Expertise to Help Improve Healthcare Access Around the World
The 'Funded Initiatives' page is entirely formal and data-driven.
Tone Analysis
Scientific Authority
Secondary Tones
- •
Empathetic
- •
Aspirational
- •
Formal
Tone Shifts
Shifts from an emotional, patient-focused tone on the homepage's cancer sections to a more corporate and informational tone in the 'Latest Articles' and 'About' sections.
A significant shift to a dense, technical, and formal tone on the 'Funded Initiatives' page, appropriate for its target audience of researchers and healthcare professionals.
Voice Consistency Rating
Good
Consistency Issues
The primary inconsistency is the sharp contrast between the highly emotional and narrative-driven voice of the main marketing pages and the very dry, functional voice of the corporate/data sections. While audience-appropriate, a stronger brand through-line could bridge this gap.
Value Proposition Assessment
Pfizer leverages its unparalleled scale, scientific expertise, and relentless R&D investment to deliver medical breakthroughs that significantly improve and extend patients' lives.
Value Proposition Components
- Component:
Leadership in Oncology
Clarity:Clear
Uniqueness:Somewhat Unique
Details:The homepage heavily promotes Pfizer's commitment to cancer research (50+ programs, 40% of R&D budget), positioning it as a primary focus.
- Component:
Massive R&D Pipeline
Clarity:Clear
Uniqueness:Unique
Details:Quantified with a 'Pipeline Snapshot' (108 total programs), demonstrating the sheer scale of its innovation engine, a key competitive advantage.
- Component:
Commitment to Global Health Equity
Clarity:Somewhat Clear
Uniqueness:Somewhat Unique
Details:Communicated through 'An Accord for a Healthier World,' though its specifics are less prominent than the oncology message.
- Component:
Patient Financial Assistance
Clarity:Clear
Uniqueness:Common
Details:Clearly articulated through 'Pfizer RxPathways,' addressing a key patient pain point, although similar programs are common among competitors.
Pfizer differentiates primarily through the scale of its operations and R&D pipeline. While competitors also focus on innovation, Pfizer’s messaging emphasizes vast numbers (50+ programs, 108 total pipeline projects, 1500 scientists) to project an image of unmatched resources and commitment. The heavy, emotive focus on oncology is a strategic choice to claim leadership in a highly visible and critical therapeutic area.
The messaging positions Pfizer as a dominant, research-driven biopharmaceutical leader that tackles the most challenging diseases. It aims to build trust through scientific authority, historical legacy (since 1849), and a demonstrated commitment to patient outcomes, particularly in cancer care. This positions them against other large pharma companies like Merck, Johnson & Johnson, and Novartis by focusing on the breadth and depth of their R&D pipeline.
Audience Messaging
Target Personas
- Persona:
Patients & Caregivers
Tailored Messages
- •
Pfizer has your back in the fight against cancer.
- •
In the fight against cancer, “one more” matters - one more wedding, one more hug, one more trial.
- •
Pfizer RxPathways connects eligible patients to a range of assistance programs...
Effectiveness:Effective
- Persona:
Healthcare Professionals (HCPs)
Tailored Messages
- •
Learn More at Let’sOutdoCancer.com/HCP
- •
Browse All Products
- •
Explore the Product Pipeline
Effectiveness:Somewhat Effective
- Persona:
Investors & Research Community
Tailored Messages
- •
Pipeline Snapshot as of August 5, 2025 (with detailed phase breakdowns)
- •
Download Complete Pipeline PDF
- •
Funded Initiatives (page with detailed grant information)
Effectiveness:Effective
- Persona:
General Public & Policymakers
Tailored Messages
- •
An Accord for a Healthier World
- •
Pfizer's Global Health Fellows Program...
- •
Starting with Charles Pfizer inventing...in 1849, our people have always been innovators...
Effectiveness:Somewhat Effective
Audience Pain Points Addressed
- •
Fear and uncertainty of a serious diagnosis (e.g., cancer)
- •
Need for life-extending treatments and hope for the future
- •
Financial burden of medications (addressed by RxPathways)
- •
Need for credible, scientifically-backed information on treatments
Audience Aspirations Addressed
- •
Living a longer, healthier life
- •
Experiencing more of life's important moments ('one more wedding, one more hug')
- •
Access to cutting-edge, innovative medical care
- •
A more equitable world for healthcare access
Persuasion Elements
Emotional Appeals
- Appeal Type:
Hope
Effectiveness:High
Examples
The fight against cancer continues with “One More”
A cancer breakthrough isn’t one moment. It’s decades of research...all with the hope of challenging what’s possible.
- Appeal Type:
Trust through Authority/Legacy
Effectiveness:High
Examples
Starting with Charles Pfizer inventing an almond-flavored antiparasite medicine in 1849...
Every product is the result of 1,500 scientists overseeing more than 500,000 lab tests...
- Appeal Type:
Social Responsibility/Altruism
Effectiveness:Medium
Examples
An Accord for a Healthier World
Pfizer's Global Health Fellows Program...
Social Proof Elements
- Proof Type:
Expert Proof (via Institutions)
Impact:Strong
Details:The 'Funded Initiatives' page lists collaborations with and grants to renowned institutions like The Children's Hospital of Philadelphia, Montefiore Medical Center, and Mount Sinai, reinforcing scientific credibility.
- Proof Type:
Data & Numbers
Impact:Strong
Details:Frequent use of large numbers to demonstrate scale and impact: '50+ programs', '80+ clinical trials', '108 Total' pipeline projects, '1,500 scientists'.
Trust Indicators
- •
Explicitly stating R&D investment figures and pipeline data.
- •
Transparency through the publication of 'Funded Initiatives' and downloadable pipeline PDFs.
- •
Highlighting the company's long history (since 1849).
- •
Showcasing partnerships with reputable medical and academic institutions.
Scarcity Urgency Tactics
None observed; this is appropriate for the industry and brand, which focuses on building long-term trust rather than immediate conversion.
Calls To Action
Primary Ctas
- Text:
Learn More at Let’sOutdoCancer.com
Location:Homepage, Oncology Section
Clarity:Clear
- Text:
Explore Pfizer Oncology
Location:Homepage, Oncology Section
Clarity:Clear
- Text:
Browse All Products
Location:Homepage, Products Section
Clarity:Clear
- Text:
Sign up now
Location:Homepage, Newsletter Section
Clarity:Clear
- Text:
Explore the Product Pipeline
Location:Homepage, Pipeline Section
Clarity:Clear
The CTAs are clear and logically placed, guiding users to relevant, deeper content. However, they are generally passive (e.g., 'Learn More,' 'Explore'). While functional, they could be more impactful by using more active, benefit-oriented language (e.g., 'See Our Pipeline of Breakthroughs,' 'Find Support for Your Prescription'). The 'Sign up now' CTA is the most direct and action-oriented.
Messaging Gaps Analysis
Critical Gaps
- •
The homepage messaging is heavily skewed towards oncology. While a strategic focus, it fails to adequately represent the breadth and importance of Pfizer's other major divisions, such as Vaccines and Internal Medicine, to a general visitor.
- •
There is a lack of a clear, prominent value proposition for potential employees or talent. The messaging is externally focused on patients and HCPs, missing an opportunity to attract top scientific talent.
- •
Messaging addressing public trust issues in the pharmaceutical industry is absent. The communication is uniformly positive, which may not resonate with a skeptical audience. Past campaigns like 'Science Will Win' successfully built trust during a crisis, and elements of that approach could be integrated.
Contradiction Points
No direct contradictions were found. However, the intense emotional appeal of the 'One More' cancer narrative could feel misaligned with the sterile, corporate presentation of other parts of the site.
Underdeveloped Areas
Storytelling around the 'people behind the science' is underdeveloped on the homepage. While the 'About' section mentions innovators, bringing the stories of the 1,500 scientists to the forefront could humanize the brand significantly.
The 'Accord for a Healthier World' is a powerful concept but its messaging feels like a secondary corporate initiative rather than a core part of the brand narrative.
Messaging Quality
Strengths
- •
Excellent use of emotional storytelling in the oncology campaign to create a powerful connection with patients and caregivers.
- •
Effectively uses data and scale (pipeline size, R&D investment) as a key differentiator and trust signal.
- •
Strong message clarity and hierarchy on the homepage, leaving no doubt about the current strategic focus on cancer.
- •
Transparently provides detailed scientific and pipeline information, reinforcing its authority and credibility with professional audiences.
Weaknesses
- •
Over-focus on oncology on the homepage diminishes the perceived importance of other multi-billion dollar business areas.
- •
The brand voice, while consistent within sections, lacks a unifying thread between the highly emotive marketing content and the formal corporate content.
- •
Calls-to-action are functional but lack persuasive, benefit-driven language.
- •
The patient journey, outside of oncology and financial assistance, is not clearly represented in the primary messaging.
Opportunities
- •
Create a more balanced homepage narrative that elevates other key focus areas (e.g., Immunology, Vaccines) alongside Oncology, perhaps through a rotating or modular hero section.
- •
Develop a content pillar around the 'Innovators at Pfizer,' featuring stories of individual scientists and researchers to humanize the R&D process.
- •
Integrate messaging around public trust and transparency more proactively, building on the legacy of campaigns like 'Science Will Win'.
- •
Segment homepage content more explicitly for different key audiences (Patients, HCPs, Investors) to create more direct and relevant user journeys from the first click.
Optimization Roadmap
Priority Improvements
- Area:
Homepage Message Hierarchy
Recommendation:Revise the homepage layout to create a more balanced representation of Pfizer's core focus areas. Feature a dynamic content block that showcases recent breakthroughs or patient stories from Oncology, Vaccines, and Immunology.
Expected Impact:High
- Area:
Brand Storytelling
Recommendation:Launch a dedicated content series, 'The People Behind the Breakthroughs,' with video interviews and articles about Pfizer scientists. Integrate these stories across the site and social channels to humanize the brand.
Expected Impact:High
- Area:
Audience Segmentation
Recommendation:Add a small, persistent navigation element or entry-point module on the homepage that explicitly directs key audiences ('For Patients', 'For Healthcare Professionals', 'For Investors') to tailored content hubs.
Expected Impact:Medium
Quick Wins
- •
A/B test CTA copy. Change passive phrases like 'Learn More' to more compelling, active phrases like 'Discover Our Science' or 'See Our Latest Research'.
- •
Elevate the 'Pfizer RxPathways' link to a more prominent position on the homepage to immediately address the critical patient need for financial assistance.
- •
Add a 'Careers' or 'Join Our Mission' link with a compelling message in the main navigation or footer to better attract scientific talent.
Long Term Recommendations
- •
Develop a comprehensive messaging framework that establishes a consistent, overarching brand voice—balancing scientific authority with human empathy—that can be adapted across all communications, from emotive campaigns to technical data sheets.
- •
Invest in interactive content to explain the drug development pipeline, making it more engaging and understandable for a broader audience, thereby increasing trust and transparency.
- •
Create a dedicated 'Trust & Transparency' section on the website that proactively addresses common questions and concerns about the pharmaceutical industry, drug pricing, and clinical trials.
Pfizer's strategic messaging on its corporate website is a powerful, though narrowly focused, vehicle for asserting its market leadership. The homepage executes a clear and deliberate strategy to position Pfizer as the dominant force in oncology, using a masterful blend of emotional storytelling ('one more hug') and data-driven authority ('50+ programs'). This effectively differentiates the brand through the sheer scale of its commitment. The messaging architecture is robust for its intended audiences, providing deep, transparent data for investors and the scientific community while offering empathetic narratives for patients. However, this intense focus on oncology comes at a cost, significantly overshadowing other vital, revenue-driving areas like vaccines and internal medicine. The brand voice, while effective within its context, shifts jarringly from highly empathetic to starkly corporate, indicating an opportunity to weave a more consistent narrative thread. Key gaps exist in messaging targeted at potential talent and in proactively addressing public skepticism about the pharmaceutical industry. To optimize, Pfizer should evolve its homepage to present a more balanced portfolio of its core strengths, humanize its scientific prowess by spotlighting the people behind the breakthroughs, and adopt more direct, action-oriented language to guide user journeys more effectively. The foundation is strong, but the messaging must broaden to fully reflect the scope and impact of the entire Pfizer enterprise.
Growth Readiness
Growth Foundation
Product Market Fit
Strong
Evidence
- •
Established portfolio of blockbuster drugs (e.g., Eliquis, Vyndaqel family) with significant global market share and robust sales.
- •
Strong recent performance, with a 10% year-over-year operational revenue increase in Q2 2025, beating expectations.
- •
Leadership in key therapeutic areas like oncology, internal medicine, vaccines, and inflammation, addressing large and persistent patient needs.
- •
Massive R&D pipeline with 108 projects, indicating a continuous effort to meet current and future market demands.
- •
Strategic acquisitions, like the $43 billion Seagen deal, have doubled the oncology pipeline to 60 programs, demonstrating a commitment to leading in high-growth areas.
Improvement Areas
- •
Mitigating the impact of the upcoming 'patent cliff,' with key drugs like Ibrance, Vyndaqel, and Eliquis facing loss of exclusivity between 2027-2029.
- •
Ensuring the successful integration of Seagen and the realization of its projected $10B+ revenue by 2030 to justify the significant investment.
- •
Improving R&D productivity to ensure the robust pipeline translates into a consistent cadence of new blockbuster approvals to offset revenue losses from expiring patents.
Market Dynamics
Approximately 5-6% CAGR, with the global pharmaceutical market projected to grow from ~$1.77 trillion in 2025 to ~$3.03 trillion by 2034.
Mature
Market Trends
- Trend:
Rise of Personalized and Precision Medicine
Business Impact:Drives the need for advanced diagnostic capabilities and targeted therapies, a key rationale for the Seagen (ADC technology) acquisition.
- Trend:
Increasing Use of AI and Machine Learning in R&D
Business Impact:Opportunity to accelerate drug discovery, shorten clinical trial timelines, and reduce R&D costs.
- Trend:
Intensifying Pricing Pressure and Regulatory Scrutiny
Business Impact:Increased pressure on margins and the need for strong health economics and real-world evidence to justify pricing to payers and governments.
- Trend:
Focus on High-Growth Therapeutic Areas
Business Impact:Strategic imperative to invest heavily in areas like oncology, immunology, and rare diseases, which Pfizer is actively pursuing.
- Trend:
Expansion into Emerging Markets
Business Impact:Significant growth vector, requiring tailored market access and commercial strategies for regions like Asia and Latin America.
Favorable, but urgent. The market is rewarding innovation in key areas where Pfizer is investing (e.g., oncology). However, the impending patent cliff creates a clear and urgent timeline for the pipeline and recent acquisitions to deliver results.
Business Model Scalability
High
Characterized by extremely high, front-loaded fixed costs (R&D, clinical trials) and relatively low variable costs for manufacturing small-molecule drugs, enabling massive operating leverage upon commercial success.
High. Once a drug is approved and commercialized, the marginal cost of producing additional units is low, leading to high-profit margins that fund future R&D.
Scalability Constraints
- •
Navigating complex, country-specific regulatory approval processes for new drugs and indications.
- •
Scaling complex manufacturing for biologics, cell, and gene therapies, which is significantly more challenging than for small molecules.
- •
Global supply chain complexity, subject to geopolitical risks and logistical challenges.
- •
Market access and reimbursement negotiations with national health systems and private payers.
Team Readiness
Very Strong. Experienced leadership team with a proven track record of executing large-scale M&A, managing a global organization, and navigating complex regulatory environments.
Pfizer is structured around key therapeutic business units (e.g., Oncology, Vaccines) which allows for focused expertise. The primary challenge is maintaining agility and fostering innovation within a massive global bureaucracy while integrating large acquisitions like Seagen.
Key Capability Gaps
- •
Deep expertise in AI/ML application for drug discovery and clinical trial optimization to compete with more digitally-native biotechs.
- •
Talent in digital therapeutics (DTx) and patient-centric digital services to create value 'beyond the pill'.
- •
Specialized manufacturing talent for next-generation modalities like cell and gene therapies.
Growth Engine
Acquisition Channels
- Channel:
Physician & Hospital Sales Force (Detailing)
Effectiveness:High
Optimization Potential:Medium
Recommendation:Integrate digital tools (e.g., remote detailing, data analytics for targeting) to enhance efficiency and effectiveness of the field force. Shift from product-focus to value-based discussions with healthcare providers.
- Channel:
Medical Science Liaisons (MSLs) & Key Opinion Leader (KOL) Engagement
Effectiveness:High
Optimization Potential:High
Recommendation:Leverage MSLs to gather real-world evidence and insights that inform R&D and market access strategies, positioning them as scientific partners rather than just educators.
- Channel:
Direct-to-Consumer Advertising (DTCA)
Effectiveness:Medium
Optimization Potential:Medium
Recommendation:Focus DTCA on new launches in competitive markets to build patient awareness and drive conversations with physicians. Utilize digital and social media channels for more targeted and measurable campaigns.
- Channel:
Payer & Formulary Access Teams
Effectiveness:High
Optimization Potential:High
Recommendation:Develop more sophisticated value propositions supported by robust health economics and outcomes research (HEOR) data to combat increasing pricing pressure and secure favorable formulary placement.
Customer Journey
The 'customer' is a complex ecosystem (Patient, Physician, Payer). The path is: Patient experiences symptoms -> Visits Physician -> Physician diagnoses and prescribes Pfizer product -> Patient gets prescription filled -> Payer provides reimbursement. Pfizer's success depends on influencing the Physician's choice and ensuring Payer access.
Friction Points
- •
Payer restrictions such as prior authorizations and step-edits.
- •
High patient co-pays or out-of-pocket costs.
- •
Competition from generics/biosimilars influencing physician and payer choice.
- •
Lack of patient awareness or education about specific conditions and treatments.
Journey Enhancement Priorities
{'area': 'Patient Support Services', 'recommendation': 'Expand and digitize patient support programs like Pfizer RxPathways to simplify access, assist with co-pays, and provide adherence support, thereby improving patient retention and outcomes.'}
{'area': 'Physician Education', 'recommendation': 'Deliver personalized, omnichannel educational content to physicians, moving beyond traditional sales calls to include webinars, digital platforms, and peer-to-peer learning to demonstrate clinical value. '}
Retention Mechanisms
- Mechanism:
Treatment for Chronic Conditions
Effectiveness:High
Improvement Opportunity:Develop and promote patient adherence programs using digital tools (apps, reminders) to improve long-term outcomes and secure recurring revenue streams.
- Mechanism:
Expanding Drug Indications
Effectiveness:High
Improvement Opportunity:Systematically pursue label expansions for existing blockbuster drugs to treat new patient populations or earlier stages of disease, thereby extending the product lifecycle and market reach.
- Mechanism:
Patient Assistance & Support Programs
Effectiveness:Medium
Improvement Opportunity:Increase awareness and ease-of-use of these programs to reduce patient drop-off due to financial barriers and build long-term patient and physician loyalty.
Revenue Economics
Extremely Favorable. For patented drugs, the price per unit is high while the marginal cost of production is low, leading to very high gross margins (reported at ~76% in Q2 2025). The primary economic challenge is recouping massive upfront R&D and clinical trial investments.
Not directly applicable in the traditional SaaS sense. A proxy would be 'Lifecycle Revenue per Drug' vs. 'Total R&D and Commercialization Cost per Drug'. For a blockbuster drug, this ratio is exceptionally high.
High, but facing pressure. Pfizer demonstrates strong revenue generation from its portfolio, but faces the constant threat of patent expiry which erases revenue streams that must be replaced by new, innovative products.
Optimization Recommendations
- •
Accelerate time-to-market for pipeline drugs to maximize the duration of patent-protected revenue generation.
- •
Optimize R&D spend by leveraging AI and partnerships to improve the success rate of clinical trials and terminate unpromising candidates earlier.
- •
Implement strategic pricing and market access initiatives to maximize revenue per product in the face of global pricing pressures.
Scale Barriers
Technical Limitations
- Limitation:
R&D Pipeline Failures
Impact:High
Solution Approach:Diversify the R&D portfolio across multiple therapeutic modalities (small molecules, biologics, ADCs, vaccines). Utilize AI and advanced analytics to improve target selection and predict trial outcomes. Pursue strategic M&A to acquire de-risked, late-stage assets.
- Limitation:
Manufacturing Complexity for Biologics
Impact:Medium
Solution Approach:Invest in 'smart manufacturing' facilities and digital supply networks to improve efficiency and mitigate operational risks for complex therapies. Partner with specialized contract development and manufacturing organizations (CDMOs) where necessary.
Operational Bottlenecks
- Bottleneck:
Global Regulatory and Reimbursement Approvals
Growth Impact:Slows down global product launches and revenue ramp-up.
Resolution Strategy:Build deep, local expertise in regulatory affairs and market access for key markets. Engage with regulators and payers early in the development process to align on evidence requirements.
- Bottleneck:
Post-Merger Integration
Growth Impact:Failure to effectively integrate large acquisitions like Seagen can lead to loss of key talent, cultural clashes, and failure to realize synergistic value.
Resolution Strategy:Establish a dedicated integration management office with clear governance and milestones. Prioritize retaining key scientific and commercial talent from the acquired company and align cultures around a shared scientific mission.
Market Penetration Challenges
- Challenge:
Patent Cliff & Generic/Biosimilar Competition
Severity:Critical
Mitigation Strategy:Aggressively defend intellectual property, develop next-generation follow-on products, and execute a robust M&A and pipeline strategy to ensure new product launches can offset the ~$200 billion industry-wide revenue at risk by 2030.
- Challenge:
Intense Competition in Key Therapeutic Areas
Severity:Major
Mitigation Strategy:Differentiate products based on clinical superiority, safety profiles, or novel mechanisms of action. Focus commercial efforts on demonstrating superior value to both clinicians and payers. Competitors include Novartis, Roche, Merck, and J&J.
- Challenge:
Drug Pricing Controls and Payer Pressure
Severity:Major
Mitigation Strategy:Generate and effectively communicate compelling real-world evidence and health economic data to prove the value of innovative medicines and justify premium pricing.
Resource Limitations
Talent Gaps
- •
Top-tier data scientists and AI/ML engineers with pharmaceutical R&D experience.
- •
Experts in cell and gene therapy manufacturing and regulation.
- •
Commercial leaders with experience in launching digital health solutions.
Significant. While generating strong cash flow, Pfizer requires immense capital for mega-deals (like the $43B Seagen acquisition), funding dozens of late-stage clinical trials simultaneously, and global product launches.
Infrastructure Needs
- •
Expansion of specialized manufacturing capacity for antibody-drug conjugates (ADCs) and other biologics.
- •
Advanced data infrastructure to support AI-driven R&D and real-world evidence analysis.
- •
Modernized, resilient global supply chain infrastructure.
Growth Opportunities
Market Expansion
- Expansion Vector:
Geographic Expansion in Emerging Markets
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Develop tiered pricing and access strategies for key emerging markets like China, India, and Brazil. Partner with local players to navigate regulatory and distribution complexities.
- Expansion Vector:
Label Expansion for Oncology Portfolio
Potential Impact:High
Implementation Complexity:Medium
Recommended Approach:Systematically conduct clinical trials to move approved cancer therapies into earlier lines of treatment (e.g., adjuvant settings) and new cancer types, significantly increasing the eligible patient population.
Product Opportunities
- Opportunity:
Leadership in Antibody-Drug Conjugates (ADCs)
Market Demand Evidence:Strong clinical data and high M&A activity in the ADC space. The Seagen acquisition positions Pfizer as a potential leader in this next-generation cancer treatment modality.
Strategic Fit:Excellent. Combines Seagen's leading ADC technology with Pfizer's global development and commercialization capabilities.
Development Recommendation:Prioritize and accelerate the development of the 12 ADCs in the combined pipeline. Explore novel ADC targets and payloads to build a sustainable long-term platform.
- Opportunity:
Obesity and Metabolic Diseases
Market Demand Evidence:Massive and rapidly growing market with high unmet need, as demonstrated by the success of competitors' GLP-1 agonists.
Strategic Fit:Aligns with the Internal Medicine focus area.
Development Recommendation:Aggressively advance pipeline candidates like danuglipron to compete in this multi-billion dollar market, focusing on differentiation (e.g., oral administration, safety profile, alternative mechanisms).
- Opportunity:
Next-Generation Vaccines (e.g., mRNA technology)
Market Demand Evidence:Proven success of mRNA platform with COVID-19 vaccines.
Strategic Fit:Core to the Vaccines business unit.
Development Recommendation:Leverage the BNTX partnership and internal mRNA expertise to develop new vaccines for infectious diseases (e.g., RSV, shingles, flu) and explore therapeutic applications in oncology and rare diseases.
Channel Diversification
- Channel:
Digital Therapeutics (DTx)
Fit Assessment:High
Implementation Strategy:Develop or acquire DTx solutions that act as companion products to key drugs (especially in oncology and immunology) to improve patient adherence, monitor side effects, and provide supportive care, creating a stickier ecosystem.
- Channel:
Direct-to-Consumer (DTC) Telehealth Platform
Fit Assessment:Medium
Implementation Strategy:Following the launch of its DTC platform, selectively expand offerings for specific conditions where patient awareness and access are key barriers. Partner with established telehealth providers to streamline the patient-physician interaction.
Strategic Partnerships
- Partnership Type:
AI/ML Drug Discovery Collaborations
Potential Partners
- •
Schrödinger
- •
Recursion Pharmaceuticals
- •
Insitro
Expected Benefits:Accelerate identification of novel drug targets and optimize molecule design, potentially reducing R&D timelines and improving the probability of success for early-stage assets.
- Partnership Type:
Early-Stage Biotech Licensing & Acquisition
Potential Partners
Numerous private and public biotech companies with promising assets in Pfizer's core therapeutic areas.
Expected Benefits:Continuously replenish the early-to-mid-stage pipeline by accessing external innovation, which is a core component of the biopharma growth model.
Growth Strategy
North Star Metric
Number of Patients Treated with New Molecular Entities (NMEs) Launched in the Last 5 Years
This metric directly measures the success of the R&D and M&A engine in delivering novel, impactful medicines to patients. It aligns with the mission of 'breakthroughs that change patients' lives' and is a leading indicator of future revenue growth, crucial for offsetting patent cliffs. Pfizer has stated a goal to double the number of patients treated with its cancer medicines by 2030.
Increase metric by 20% annually over the next five years, driven by successful launches from the Seagen acquisition and the internal pipeline.
Growth Model
Innovation-Driven Growth, Fueled by Strategic M&A
Key Drivers
- •
R&D Productivity & Pipeline Advancement
- •
Successful Integration and Commercialization of Acquired Assets (especially Seagen)
- •
Maximizing Lifecycle Value of Existing Blockbusters through Label Expansion
- •
Securing Global Market Access and Reimbursement
A dual-pronged approach: 1) Invest heavily in internal R&D in core areas of expertise (e.g., small molecules, vaccines). 2) Aggressively pursue business development and M&A to acquire new technologies and late-stage assets to fill portfolio gaps and counteract patent expirations.
Prioritized Initiatives
- Initiative:
Accelerate and Integrate the Seagen Oncology Portfolio
Expected Impact:High
Implementation Effort:High
Timeframe:18-24 months for full integration and pipeline acceleration
First Steps:Finalize integrated R&D and commercial leadership teams. Harmonize clinical trial protocols for key ADC assets. Develop a unified commercial launch strategy for late-stage candidates.
- Initiative:
Advance Key Late-Stage Pipeline Assets Outside of Oncology
Expected Impact:High
Implementation Effort:Medium
Timeframe:12-36 months to key clinical readouts/regulatory filings
First Steps:Fully fund and staff pivotal Phase 3 trials for promising candidates in immunology, obesity, and vaccines. Engage with regulatory agencies to align on endpoints and filing requirements.
- Initiative:
Launch Enterprise-Wide AI in R&D Program
Expected Impact:Medium-High (long-term)
Implementation Effort:High
Timeframe:24+ months to see significant impact on pipeline velocity
First Steps:Appoint a Head of AI for R&D. Identify 2-3 high-impact pilot projects in drug discovery or clinical trial design. Secure partnerships with leading AI technology firms.
Experimentation Plan
High Leverage Tests
- Area:
Commercial Model Innovation
Experiment:Pilot an outcome-based pricing model with a regional payer for a new high-cost therapy.
- Area:
Digital HCP Engagement
Experiment:Run an A/B test comparing the impact of a fully digital engagement model vs. traditional sales rep model for a mature product in a specific geography.
- Area:
Clinical Trial Recruitment
Experiment:Utilize a decentralized clinical trial (DCT) platform for a Phase 2 study to measure impact on recruitment speed and patient diversity.
For commercial tests, track physician prescribing behavior, market share, and ROI. For R&D experiments, track metrics like cycle time, cost per patient, and data quality.
Continuous experimentation in commercial models; project-based for clinical trial innovations.
Growth Team
Decentralized 'Asset-Centric' Growth Teams. Each major drug or late-stage asset should have a dedicated, cross-functional team comprising R&D, Medical Affairs, Market Access, Commercial, and Regulatory leads empowered to drive the asset's global strategy and execution.
Key Roles
- •
Global Asset Lead (overall accountability for the drug's lifecycle)
- •
Head of Health Economics & Outcomes Research (to build the value story)
- •
Digital Marketing & Patient Engagement Lead
- •
Head of AI for a specific Therapeutic Area
Invest in continuous training for commercial and medical teams on health economics, real-world evidence, and digital engagement. Establish a reverse-mentoring program where junior, digitally-native employees are paired with senior leaders.
Pfizer stands at a pivotal moment, possessing a strong growth foundation but facing critical challenges that will define its trajectory through 2030. The company's product-market fit is undeniable, anchored by a portfolio of globally recognized blockbuster drugs and a robust R&D pipeline. The market dynamics are favorable, with a growing global demand for innovative medicines, particularly in Pfizer's focus areas of oncology, immunology, and vaccines. The business model is highly scalable, and the leadership team is exceptionally experienced in navigating the complexities of the biopharmaceutical landscape.
The primary growth engine is a powerful combination of internal R&D and aggressive, strategic M&A. The recent $43 billion acquisition of Seagen is a bold, transformative bet that doubles down on oncology and positions Pfizer to lead the next wave of cancer therapy with antibody-drug conjugates (ADCs). This move, alongside other acquisitions, is the central pillar of the strategy to overcome the most significant barrier to its growth: the impending patent cliff. Between 2027 and 2029, Pfizer faces the loss of exclusivity for several key revenue drivers, creating an urgent need for its pipeline and newly acquired assets to deliver commercially successful products.
Key growth opportunities are clear. First, dominating the ADC space by successfully integrating Seagen and accelerating its pipeline. Second, capitalizing on other high-potential therapeutic areas like obesity and next-generation vaccines. Third, leveraging digital and AI to enhance R&D productivity and create more personalized engagement with physicians and patients. However, scaling is not without barriers. Intense competition, relentless pricing pressure from payers, and the inherent risks of drug development mean that execution must be flawless.
Strategic Recommendations:
-
Laser-Focus on Oncology Execution: The success or failure of the Seagen integration will be the defining story of the next five years. Pfizer must prioritize retaining key talent, accelerating the combined pipeline, and establishing clear market leadership in ADCs. The goal of having at least eight oncology blockbusters by 2030 is ambitious but necessary.
-
Diversify the Innovation Engine: While oncology is the centerpiece, Pfizer must continue to advance its most promising assets in other areas, such as the oral GLP-1 candidate for obesity, to avoid over-concentration in a single, highly competitive therapeutic area.
-
Embed AI and Digital Across the Value Chain: Move beyond pilot projects to a scaled, enterprise-wide adoption of AI in R&D to shorten timelines and increase the probability of success. Simultaneously, invest in digital patient support and physician engagement platforms to build moats around key products.
Pfizer's growth readiness is strong, but its future success is contingent on a period of intense and successful execution. The strategy is sound, the assets are in place, but the race against the patent clock is on.
Legal Compliance
Pfizer maintains a comprehensive and multi-layered approach to privacy, with a detailed global Privacy Policy and specific notices for different jurisdictions (e.g., California, EU). The policy is easily accessible and outlines the types of personal data collected, its usage, and data protection measures. It emphasizes user rights and transparency. The policy explicitly covers data sharing with subsidiaries, partners, and third-party service providers, stating that such sharing is governed by the terms of the privacy policy. For data transfers, particularly from regions like the EU or Saudi Arabia, Pfizer states it will handle data in accordance with its policy and employ appropriate security measures, though it notes that data may be stored in the U.S. where protection levels might differ. The policy also details the use of tracking technologies like cookies and web beacons.
Pfizer's Terms of Use are clearly articulated, covering user obligations, intellectual property rights (protecting trademarks like the Pfizer name and logo), and limitations of liability. The terms state that user access is at their own risk and disclaim warranties to the fullest extent permitted by law. They include a standard indemnification clause requiring users to hold Pfizer harmless from losses resulting from violations of the terms. A key clause, subject to the privacy policy, deems any user-submitted communication (questions, comments) as non-confidential, granting Pfizer broad rights to use such information. The governing law is specified as that of the United States and the State of New York.
The website provides a 'Cookies Preference' link, indicating a mechanism for user consent. The privacy policy explains the use of cookies, log files, and pixel tags for personalization and usage tracking. Pfizer's policy notes that users can control cookie acceptance through their browser settings, but warns that rejecting cookies may impair site functionality. It also acknowledges the use of cookies by third-party partners (e.g., Google Analytics) and states that while they don't want partners tracking users off-site, they may not have total control over it. This suggests a good-faith effort towards transparency, but the reliance on browser settings rather than a granular, on-site consent tool for all jurisdictions could be a gap in regions with strict opt-in laws.
Pfizer demonstrates a strong commitment to data protection, framed as a fundamental right. They outline principles of respect, transparency, appropriate use, and safeguarding of data. Technical and organizational controls, including firewalls and physically secure data centers, are in place to prevent unauthorized access. Specific notices, like the one for EU/EEA citizens regarding their Compliance Helpline, detail the legal basis for processing data under GDPR (legal obligation, legitimate interest). For sensitive health information collected through programs like co-pay assistance, Pfizer obtains explicit consent outlining what is collected, why, and for how long. They also have a clear process for individuals to exercise their data rights.
The website shows evidence of attention to accessibility, such as the 'Skip to main content' link on its pages, a key feature for users of screen readers. Pfizer has a formal internal policy regarding the Americans with Disabilities Act (ADA) for its employees and applicants, indicating corporate-level awareness of accessibility requirements. However, a publicly visible Accessibility Statement detailing commitment to standards like the Web Content Accessibility Guidelines (WCAG) was not found in the provided content or initial search. While the 'skip link' is a positive indicator, a comprehensive WCAG audit would be necessary to fully assess compliance with standards like WCAG 2.1 AA, which are the de facto legal benchmark for ADA compliance in the digital realm.
Pfizer's legal positioning is heavily defined by stringent pharmaceutical industry regulations.
1. Marketing and Promotion (FDA & EMA): All website content promoting products must maintain a 'fair balance' of benefit and risk information. Direct-to-consumer advertising for prescription drugs is prohibited in the EU but permitted in the U.S., subjecting Pfizer's U.S.-facing content to strict FDA oversight regarding claims, risk disclosure, and non-misleading information.
2. Transparency (Sunshine Act): The 'Funded Initiatives' page is a direct manifestation of compliance with transparency laws like the U.S. Physician Payments Sunshine Act, which mandates the public disclosure of financial relationships with healthcare professionals and organizations. This transparency builds trust and mitigates risks of perceived improper influence.
3. Data Privacy (HIPAA & GDPR): While the main corporate site may not directly process Protected Health Information (PHI), patient support programs like RxPathways and co-pay assistance programs collect sensitive health data, requiring robust controls and explicit consent, bordering on HIPAA-level considerations even if not directly governed by it. GDPR compliance is explicitly addressed with a designated Data Protection Officer.
4. Anti-Corruption (FCPA): As a global company, Pfizer is subject to the Foreign Corrupt Practices Act. Their extensive compliance program, policies, and history demonstrate a deep engagement with anti-bribery and anti-corruption standards in their interactions with government officials and healthcare providers globally.
Compliance Gaps
- •
Absence of a clearly visible, dedicated 'Accessibility Statement' on the website homepage, which could detail commitment to WCAG 2.1 AA standards.
- •
The cookie consent mechanism appears to rely heavily on user browser settings rather than a prominent, granular opt-in banner required by GDPR and other modern privacy laws before any non-essential cookies are placed.
- •
Lack of a 'Do Not Sell or Share My Personal Information' link in the website footer, which is a specific requirement under the CCPA/CPRA for California residents.
Compliance Strengths
- •
Robust and multi-layered privacy policy framework with jurisdiction-specific notices (e.g., for California HCPs) that enhances clarity and legal resilience.
- •
Excellent transparency regarding financial relationships with the healthcare community through the 'Funded Initiatives' page, directly aligning with Sunshine Act requirements and building public trust.
- •
Strong, well-documented internal compliance programs and codes of conduct (e.g., 'Blue Book', 'White Guide') covering key risk areas like anti-corruption, ethical marketing, and healthcare law.
- •
Clear and enforceable Terms of Use that protect intellectual property and limit liability effectively.
- •
Explicit consent mechanisms for collecting sensitive health information in patient support programs, demonstrating a high degree of care with sensitive data.
Risk Assessment
- Risk Area:
FDA/EMA Marketing Compliance
Severity:High
Recommendation:Continuously audit all product-related website content, including articles and pipeline information, to ensure strict adherence to 'fair balance' principles, presenting risks as prominently as benefits. Implement a rigorous internal review process for all web content updates to prevent any statements that could be construed as promoting off-label use.
- Risk Area:
Cookie Consent and Tracking
Severity:Medium
Recommendation:Implement a leading third-party consent management platform (CMP) to provide a clear, granular, and geographically-aware cookie consent banner. This banner should block all non-essential scripts and cookies prior to obtaining user consent to ensure full compliance with GDPR's opt-in requirements.
- Risk Area:
Website Accessibility (ADA/WCAG)
Severity:Medium
Recommendation:Conduct a formal website accessibility audit against WCAG 2.1 AA standards using a combination of automated tools and manual testing by users with disabilities. Publish an official Accessibility Statement on the website, outlining the company's commitment and providing a channel for users to report issues.
- Risk Area:
CCPA/CPRA Compliance
Severity:Low
Recommendation:Add a 'Do Not Sell or Share My Personal Information' link to the website footer to comply with California privacy law requirements, even if the company's position is that it does not 'sell' data in the traditional sense. This provides a clear and compliant path for consumers to exercise their rights.
High Priority Recommendations
- •
Immediately review and enhance the cookie consent mechanism to ensure it meets the strict 'prior consent' opt-in standards of GDPR, as this represents a significant and easily identifiable compliance gap for EU visitors.
- •
Commission a third-party audit of the website against WCAG 2.1 AA standards and publish an Accessibility Statement to mitigate legal risk under the ADA and improve usability for all patients.
- •
Maintain vigilant oversight of all marketing and product-related content to ensure it strictly adheres to FDA 'fair balance' requirements, given the high financial and reputational stakes of regulatory enforcement action.
Pfizer's legal positioning, as reflected by its corporate website, is exceptionally mature and robust, which is expected of a leader in the highly regulated biopharmaceutical industry. The company demonstrates a sophisticated understanding of its complex legal obligations across data privacy, marketing, and transparency. Strengths are particularly evident in its detailed, multi-jurisdictional privacy policies and its proactive transparency regarding payments to healthcare professionals, which aligns with the U.S. Sunshine Act and fosters significant public trust. The internal compliance frameworks, such as the 'White Guide,' underscore a deep, process-oriented approach to mitigating legal risk.
However, there are tactical gaps in its digital execution. The most notable is the website's cookie consent mechanism, which appears less stringent than the explicit, prior-consent model required by GDPR. Additionally, while showing some good accessibility practices (e.g., 'skip to content' links), the lack of a formal, public Accessibility Statement and a clearly visible link for California residents to opt-out of data sharing represent missed opportunities to solidify its compliance posture and enhance user trust. Addressing these digital interface issues would further strengthen an already formidable legal and compliance framework, enhancing its strategic value as a business asset that facilitates market access and reinforces its reputation for integrity.
Visual
Design System
Modern Corporate & Scientific
Excellent
Advanced
User Experience
Navigation
Horizontal Top-Level with Mega Menus
Intuitive
Excellent
Information Architecture
Logical
Clear
Light
Conversion Elements
- Element:
Homepage 'In the fight against cancer' CTA
Prominence:High
Effectiveness:Effective
Improvement:The button label 'Our work in cancer' is clear, but could be more action-oriented. Consider testing a label like 'Explore Our Cancer Research' to create a stronger sense of active discovery.
- Element:
Footer 'Sign up for communications from Pfizer' form
Prominence:Medium
Effectiveness:Somewhat effective
Improvement:The CTA is placed very low on the page. To increase newsletter sign-ups, consider adding this module higher up on relevant pages, such as news or research sections, or using a less intrusive, sticky footer element.
- Element:
Search bar 'Looking for a product?'
Prominence:Medium
Effectiveness:Effective
Improvement:The search functionality is good. To enhance it, implement auto-suggest with product names, therapeutic areas, or research topics as the user types to speed up information discovery.
- Element:
Funded Initiatives search/filter form
Prominence:High
Effectiveness:Effective
Improvement:The form is comprehensive but visually dense. Use visual dividers or group related fields under subheadings (e.g., 'Project Details', 'Date Range') to improve scannability and reduce the initial cognitive load for users.
Assessment
Strengths
- Aspect:
Cohesive Brand Identity
Impact:High
Description:The website consistently uses Pfizer's new brand identity, centered around the helix logo and a vibrant two-tone blue palette. This reinforces the company's shift towards being a science-driven, innovative biopharmaceutical leader. The design feels modern, trustworthy, and professional, aligning with the company's mission.
- Aspect:
Clear Visual Hierarchy & Scannability
Impact:High
Description:The homepage effectively uses size, color, and whitespace to guide the user's eye. Large hero imagery, bold headlines, and succinct text blocks create a clear path through the content. Key sections like 'Latest Articles' and therapeutic areas are well-defined and easy to parse.
- Aspect:
Intuitive Navigation & Information Architecture
Impact:High
Description:The primary navigation is simple and logically organized around key audience needs (Science, Products, Stories, etc.). This clear structure makes it easy for diverse audiences like patients, healthcare professionals (HCPs), and investors to find relevant information quickly.
- Aspect:
High-Quality Visual Storytelling
Impact:Medium
Description:The use of compelling, high-quality imagery and video effectively humanizes the brand and its scientific work. The hero video on the homepage, for example, connects the company's research to its ultimate purpose: improving patient lives.
Weaknesses
- Aspect:
Data-Heavy Presentation
Impact:Medium
Description:The 'Funded Initiatives' page is essentially a wall of data. While the information is valuable, the pure table-based layout is visually overwhelming and difficult to scan. This can lead to user fatigue and abandonment for those seeking specific information.
- Aspect:
Understated Calls-to-Action
Impact:Low
Description:While CTAs exist, they are often styled as simple text links or understated buttons (e.g., 'Learn More'). More prominent, action-oriented CTAs with stronger visual contrast could improve user engagement and guide users more effectively through conversion funnels.
- Aspect:
Lack of Interactive Data Visualization
Impact:Medium
Description:The section showcasing statistics (46 programs, 30 breakthroughs, etc.) uses static numbers. This is a missed opportunity for engagement. Interactive charts or graphs could make this data more compelling and memorable.
Priority Recommendations
- Recommendation:
Incorporate Interactive Data Visualization
Effort Level:Medium
Impact Potential:High
Rationale:Transform the static table on the 'Funded Initiatives' page into a filterable and sortable interactive dashboard. Add visual elements like charts or maps to represent the data, making it more digestible and engaging for researchers, partners, and the public.
- Recommendation:
Enhance CTA Prominence and Language
Effort Level:Low
Impact Potential:Medium
Rationale:Conduct A/B testing on key CTA buttons. Test variations in color, size, and microcopy (e.g., 'Explore Our Science' vs. 'Learn More'). A small change can significantly increase click-through rates and guide users towards key strategic content.
- Recommendation:
Develop Audience-Specific Content Hubs
Effort Level:High
Impact Potential:High
Rationale:While navigation is clear, creating dedicated content hubs for 'Patients & Caregivers' and 'Healthcare Professionals' would further tailor the user experience. These hubs can aggregate the most relevant articles, tools, and resources, reducing the number of clicks required to find critical information, a noted best practice for pharma websites.
Mobile Responsiveness
Excellent
The design appears well-structured to adapt cleanly to various breakpoints. The use of a card-based layout and a clear grid system on the desktop version suggests that content blocks will stack logically and maintain readability on tablet and mobile screens.
Mobile Specific Issues
The main navigation will likely collapse into a hamburger menu, which is standard practice but requires an extra tap to access main site sections.
Data-heavy tables, like the one on the 'Funded Initiatives' page, will be challenging to navigate on small screens and may require horizontal scrolling, which is a poor user experience.
Desktop Specific Issues
Large, high-resolution images and videos may contribute to longer page load times on slower connections, impacting the initial user experience.
Pfizer's website is a world-class example of a modern corporate digital presence that successfully communicates its brand identity as a leader in biopharmaceutical science. The recent rebranding is executed flawlessly across the site, with the helix motif and a consistent blue color palette reinforcing the company's core mission: 'Breakthroughs that change patients' lives.'
Design System and Brand Identity:
The visual design is clean, professional, and highly consistent. The design system is clearly mature, utilizing a well-defined grid, typography scale, and component library (buttons, cards, etc.). This ensures a cohesive experience across different pages and sections. The use of the Noto Sans typeface, chosen for its global accessibility across hundreds of languages, is a strategic choice that aligns with Pfizer's global reach. The balance of scientific imagery (microscopic views of cells) with human-centric photography (patients and researchers) effectively communicates both the company's technical expertise and its ultimate purpose.
Visual Hierarchy and User Experience:
The information architecture is logical and intuitive, catering to a diverse audience that includes patients, healthcare providers, investors, and the general public. The homepage does an excellent job of establishing a visual hierarchy that guides users to key areas. The large, impactful hero section immediately grabs attention, followed by clearly demarcated content blocks for news, research highlights, and corporate information. The main navigation is streamlined, using clear labels that avoid industry jargon, which lowers the cognitive load for non-expert users.
The 'Funded Initiatives' page, however, represents a significant UX weakness. It presents a vast amount of valuable data in a dense, static table. This format is not user-friendly, especially for users trying to find specific information quickly. It lacks the scannability and interactive elements expected of a modern, data-rich web page.
Conversion and Engagement:
As a pharmaceutical corporate site, traditional e-commerce conversions are not the primary goal. Instead, 'conversion' is about engaging key audiences and guiding them to information. Key conversion elements include the newsletter sign-up, search functionality for products and research, and CTAs that lead to deeper content. The primary CTAs are effective but could be optimized. They are visually clear, but their language is often passive ('Learn more'). Adopting more active, compelling language could improve engagement.
Visual Storytelling:
The site excels at visual storytelling. The homepage hero video and the 'Stories' section use powerful visuals to connect the company’s complex scientific work to tangible human outcomes. This approach is critical for building trust and making the brand's purpose relatable. The way the site showcases its therapeutic areas—Internal Medicine, Inflammation & Immunology, Vaccines, and Oncology—with abstract, beautiful, and color-coded microscopic imagery is a particularly strong example of translating complex science into an accessible and aesthetically pleasing visual language.
In conclusion, Pfizer's website is a visually impressive and strategically sound digital platform. Its primary strengths lie in its cohesive brand expression and clear information architecture. The most significant opportunity for improvement lies in enhancing the presentation of data-heavy content, transforming static tables into interactive and user-centric experiences.
Discoverability
Market Visibility Assessment
Pfizer commands exceptional brand authority, significantly amplified by its leadership role in developing the COVID-19 vaccine. This has translated into household-name status and high public trust. Its digital presence reinforces this through a strong emphasis on 'breakthroughs,' deep scientific content, a transparent R&D pipeline, and a clear focus on high-impact disease areas like oncology. The 'Funded Initiatives' page is a standout asset, providing unparalleled transparency into its research grants, which solidifies its authority and role as a central pillar in the broader healthcare ecosystem.
Pfizer competes digitally with other pharmaceutical giants like Johnson & Johnson, Merck, Roche, and Novartis for visibility in key therapeutic areas. The homepage's focus on oncology is a clear strategic choice to dominate search visibility in this highly competitive and lucrative market, where it is a top global player. Similarly, its focus on vaccines, internal medicine, and immunology content aligns directly with its primary revenue drivers and R&D pipeline, aiming to capture the attention of both patients and Healthcare Professionals (HCPs) searching for information in these fields.
For a biopharmaceutical leader, 'customer acquisition' translates to patient and HCP engagement. Pfizer's website effectively targets both audiences. For patients, it offers educational content on diseases and direct access to patient assistance via 'Pfizer RxPathways.' For HCPs, it provides in-depth scientific data, a detailed product pipeline, and research-focused content hubs ('Focus Areas'). The digital strategy aims to inform patient-doctor conversations and establish Pfizer as an essential resource for medical professionals, which is critical for treatment adoption.
The primary .com website is global in scope but primarily tailored to the U.S. market and English-speaking audiences. While initiatives like 'An Accord for a Healthier World' and articles on global health signal a worldwide mission, significant opportunity exists to enhance market penetration through more dedicated, localized content strategies for other major international markets, adapting to regional languages, regulations, and healthcare priorities.
Pfizer's digital presence demonstrates comprehensive expertise across its core focus areas: oncology, vaccines, internal medicine, and immunology. The site is structured to guide users to deep, specialized content, from its product pipeline and clinical trial data to articles on global health initiatives. The 'Funded Initiatives' section is a unique asset, providing a searchable database of grants that showcases the breadth of its impact on medical research and elevates its demonstrated expertise far beyond its own commercial products.
Strategic Content Positioning
Content is well-structured to meet the needs of different audiences at various stages. High-level corporate news and purpose-driven stories build brand awareness. Detailed sections on diseases and therapeutic areas serve the consideration and research phases for both patients and HCPs. Deeply scientific content, such as pipeline data and clinical trial information, is crucial for the decision-making and trust-building stage with prescribing physicians and the research community. Patient support programs like RxPathways directly address affordability concerns, a key part of the patient journey.
Pfizer is already a thought leader, but it can further amplify its position by transforming its vast data assets into more accessible content formats. The 'Funded Initiatives' database is a prime example—it could be leveraged to create annual reports, data visualizations, and expert analyses on medical research trends. This would generate significant media attention and high-authority backlinks, positioning Pfizer not just as a drug maker, but as a key source of industry intelligence.
While competitors also provide extensive scientific content, Pfizer's key differentiator is its transparency, particularly through the 'Funded Initiatives' page. A strategic opportunity lies in creating more patient-centric content that translates complex scientific breakthroughs into understandable, empowering narratives. While the science is strong, content that addresses the emotional and logistical journey of a patient could create a deeper, more resonant connection.
The core message, 'Breakthroughs that change patients’ lives,' is consistently and effectively communicated across the website. This is reinforced by the heavy emphasis on R&D, the detailed pipeline snapshot, patient support programs, and global health initiatives. The prominent focus on oncology on the homepage aligns with its strategic goal of market leadership in cancer care.
Digital Market Strategy
Market Expansion Opportunities
- •
Develop dedicated, localized content hubs for key international markets to deepen geographic penetration.
- •
Create comprehensive, patient-friendly digital resource centers for major diseases within focus areas (e.g., a 'Breast Cancer Hub') to capture search traffic across the entire patient journey.
- •
Leverage the 'Funded Initiatives' data to create targeted content for specific research communities, fostering collaboration and solidifying authority in emerging therapeutic areas.
Customer Acquisition Optimization
- •
Create a dedicated, gated portal for HCPs offering exclusive access to research data, webinars with Pfizer scientists, and advanced clinical trial information to build a loyal professional audience.
- •
Simplify the user path to patient assistance programs like RxPathways, using clear calls-to-action on all relevant disease and product pages to reduce friction.
- •
Implement personalized content journeys for returning visitors based on their browsing behavior (e.g., showing more oncology content to a user who has previously visited cancer-related pages).
Brand Authority Initiatives
- •
Launch a major content marketing campaign centered around the 'Funded Initiatives' database, promoting it as a free, valuable tool for the entire medical research community.
- •
Establish a high-profile 'Science at Pfizer' blog or video series featuring top executives and scientists discussing future medical trends and innovations.
- •
Actively syndicate data-driven insights and reports based on Pfizer's research to top-tier medical and news publications to earn high-authority media mentions and backlinks.
Competitive Positioning Improvements
- •
Position Pfizer as the leader in transparency within Big Pharma by more actively promoting its open data initiatives.
- •
Double down on the 'Oncology' focus in digital marketing to build an insurmountable search visibility lead against competitors like Roche and Merck for cancer-related terms.
- •
Use digital storytelling to highlight the human impact of Pfizer's breakthroughs, shifting the brand narrative from a corporate giant to a patient-centric innovator.
Business Impact Assessment
Success is measured by share of voice and search visibility for high-value, non-branded keywords within strategic focus areas (e.g., 'next-generation biologics,' 'new treatments for metastatic breast cancer') relative to key competitors such as Merck, Roche, Johnson & Johnson, and AbbVie.
For HCPs, key metrics include engagement rates with scientific content, clinical trial data downloads, and registrations for professional webinars. For patients, metrics center on traffic to disease education pages, video engagement, and inquiries or applications for patient support programs like RxPathways.
Authority is measured by the quantity and quality of backlinks from medical institutions, universities, and major news outlets. Other key indicators include growth in organic search traffic for research-level keywords, branded search volume, and positive sentiment in media mentions related to innovation and transparency.
Benchmarking involves regular analysis of search engine rankings and share of voice for a defined set of strategic keywords against a primary competitor set. This also includes comparing content depth, audience engagement on shared platforms, and the frequency of media citations for research and innovation.
Strategic Recommendations
High Impact Initiatives
- Initiative:
Weaponize Transparency: The 'Funded Initiatives' Content Hub
Business Impact:High
Market Opportunity:Positions Pfizer as the undisputed leader in R&D transparency, building immense trust with the medical community and creating a powerful, link-worthy asset that competitors cannot easily replicate.
Success Metrics
- •
Number of backlinks from .edu and .gov domains
- •
Media mentions citing the database
- •
Referral traffic from academic and research institutions
- •
Improved search rankings for 'pharmaceutical research' terms
- Initiative:
Launch Dedicated Patient Journey Resource Centers
Business Impact:High
Market Opportunity:Captures a significant share of search traffic from patients and caregivers at all stages of their journey, from diagnosis to treatment management. This builds brand preference and directly supports patients who may eventually use Pfizer products.
Success Metrics
- •
Organic traffic growth to disease-specific sections
- •
Time on page and engagement rates with patient content
- •
Click-through rates to patient support (RxPathways)
- •
Top search rankings for long-tail patient queries
- Initiative:
Develop an Exclusive HCP Digital Engagement Portal
Business Impact:Medium
Market Opportunity:Creates a direct, high-value communication channel with the core audience of prescribing physicians, fostering loyalty and providing a competitive advantage in disseminating new clinical data and product information.
Success Metrics
- •
HCP registration and active user rates
- •
Consumption of gated content (downloads, webinar views)
- •
Engagement within the portal's community features
- •
Positive feedback from HCPs via surveys
Shift Pfizer's digital brand identity from a 'producer of medicines' to a 'central nervous system of medical innovation.' This will be achieved by leveraging its unparalleled data and research funding transparency to become the go-to authority for not only its own products but also broader trends in medical science. The strategy is to win the trust of HCPs and the respect of the public by being the most open, data-forward, and scientifically rigorous presence in the biopharmaceutical industry.
Competitive Advantage Opportunities
- •
Establish an unassailable authority position through data transparency, making it difficult for less transparent competitors to compete on trust.
- •
Build direct, defensible relationships with HCPs through a value-added digital portal, reducing reliance on traditional sales channels.
- •
Dominate the search landscape for key, high-margin therapeutic areas like oncology, becoming the first click for patients, caregivers, and professionals seeking information.
Pfizer's digital market presence is a formidable asset, characterized by high brand authority, comprehensive content, and a clear strategic focus on its core business pillars, particularly oncology. The website effectively serves its dual audiences—patients and healthcare professionals (HCPs)—by providing distinct pathways to relevant information, from high-level brand narratives to granular scientific data.
The most significant untapped opportunity lies in weaponizing its transparency. The 'Funded Initiatives' page is more than a simple disclosure; it is a strategic asset that can be transformed into a world-class content hub. By building data visualizations, trend reports, and expert analysis around this unique dataset, Pfizer can position itself as an indispensable resource for the entire medical ecosystem, generating unparalleled authority and high-quality backlinks that competitors cannot match.
Strategically, Pfizer should deepen its focus on the end-to-end user journey. For patients, this means creating comprehensive, empathetic resource centers that guide them from diagnosis through treatment. For HCPs, it involves building an exclusive digital community that provides undeniable value beyond product information, fostering long-term loyalty.
By executing on these initiatives, Pfizer can leverage its digital presence to create a durable competitive advantage built on trust, authority, and unparalleled scientific depth, solidifying its market leadership for the next decade.
Strategic Priorities
Strategic Priorities
- Title:
Drive Oncology Market Leadership via Accelerated Seagen Integration and ADC Platform Dominance
Business Rationale:The $43B Seagen acquisition is the cornerstone of Pfizer's strategy to offset the looming ~$17B patent cliff. Flawless and rapid integration is critical to capture the projected $10B+ in 2030 revenues and establish a durable, high-growth engine for the next decade.
Strategic Impact:This initiative pivots Pfizer's core business from reliance on expiring small molecules to leadership in next-generation biologics. It aims to create an unassailable leadership position in oncology, the largest and fastest-growing therapeutic area, securing the company's long-term growth trajectory.
Success Metrics
- •
Revenue from the Seagen portfolio
- •
Time-to-market for key Antibody-Drug Conjugate (ADC) assets
- •
Number of launched oncology blockbusters (target: 8+ by 2030)
- •
Retention rate of key Seagen scientific talent
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Market Position
- Title:
Launch an Enterprise-Wide AI Initiative to Reinvent R&D Productivity
Business Rationale:The traditional biopharma R&D model is too slow and costly to consistently outpace patent expirations. A systematic, top-down integration of AI and machine learning is required to shorten discovery timelines, improve clinical trial success rates, and maximize return on R&D investment.
Strategic Impact:This transforms R&D from a cost center into a sustainable competitive advantage. It will accelerate the delivery of new medicines, improve capital allocation by terminating unpromising candidates earlier, and position Pfizer as a leader in tech-enabled drug discovery.
Success Metrics
- •
Reduction in average drug discovery-to-Phase 1 timeline
- •
Increase in clinical trial success rate (Phase 2 to 3)
- •
Number of novel drug targets identified via AI platforms
- •
Projected Net Present Value (NPV) of the AI-influenced pipeline
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Operations
- Title:
Establish an Integrated Digital Health Ecosystem to Drive Patient Adherence and Outcomes
Business Rationale:The market is shifting from selling pharmaceuticals to delivering holistic health outcomes. By building a 'beyond the pill' ecosystem of digital therapeutics, diagnostics, and patient support services, Pfizer can improve medication adherence, create defensible moats around its products, and build direct patient relationships.
Strategic Impact:This strategy evolves the business model from being a drug manufacturer to a comprehensive healthcare solutions partner. It increases the lifetime value of patients, improves real-world efficacy of its core products, and creates a sticky ecosystem that is difficult for competitors to replicate.
Success Metrics
- •
Measurable improvement in patient adherence rates for key therapies
- •
Number of patients actively using companion digital health platforms
- •
Revenue generated from digital therapeutic or diagnostic solutions
- •
Inclusion of integrated solutions in value-based care agreements with payers
Priority Level:MEDIUM
Timeline:Long-term Vision (12+ months)
Category:Customer Strategy
- Title:
Secure a Leadership Position in a Second High-Growth Market: Obesity
Business Rationale:While oncology is the primary focus, over-concentration is a strategic risk. The multi-billion dollar obesity market represents the most significant near-term opportunity to diversify revenue. Aggressively advancing pipeline candidates is critical to capture a share of this explosive growth and balance the portfolio.
Strategic Impact:Establishes a second major growth pillar for the post-2030 era, mitigating risks associated with the hyper-competitive oncology market. A successful entry would create a new blockbuster revenue stream, providing a crucial buffer against patent cliffs and pipeline setbacks.
Success Metrics
- •
Successful completion of Phase 3 trials for oral GLP-1 candidate
- •
Time-to-market relative to competitors
- •
Market share captured within 3 years of launch
- •
Demonstrated differentiation on administration (oral) or safety profile
Priority Level:MEDIUM
Timeline:Strategic Initiative (3-12 months)
Category:Market Position
- Title:
Pioneer Value-Based Commercial Models to Proactively Counter Global Pricing Pressures
Business Rationale:Increasing government pricing controls, such as the US Inflation Reduction Act (IRA), represent a significant and growing threat to revenue and margins. A proactive strategy to lead the industry in developing and scaling outcome-based pricing models is essential for defending the value of innovation.
Strategic Impact:This initiative shifts Pfizer from a defensive, price-taking position to a leadership role in defining the future of pharmaceutical value. It protects long-term profitability, strengthens partnerships with payers, and creates a more predictable and sustainable commercial environment.
Success Metrics
- •
Number of value-based or outcome-based contracts signed with major payers
- •
Successful pricing negotiations for key products under new regulatory frameworks
- •
Improved or maintained gross-to-net revenue ratio for innovative therapies
- •
Positive sentiment and case studies from payer partners
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Revenue Model
Pfizer must rapidly transform from a diversified pharmaceutical giant into a science-driven, oncology-focused leader, leveraging the Seagen acquisition to build a dominant franchise that more than offsets its imminent patent cliff. This transformation requires reinventing R&D productivity through AI and establishing new growth platforms in high-value markets and digital health.
Unmatched global R&D and commercialization scale, supercharged by a leading next-generation technology platform in Antibody-Drug Conjugates (ADCs).
The successful integration of Seagen and the rapid advancement of its combined, best-in-class oncology pipeline to deliver multiple new blockbuster medicines before 2030.