eScore
regeneron.comThe eScore is a comprehensive evaluation of a business's online presence and effectiveness. It analyzes multiple factors including digital presence, brand communication, conversion optimization, and competitive advantage.
Regeneron's digital presence is authoritative and professional, excelling in content authority through its science-first messaging and detailed information on the Regeneron Genetics Center (RGC). Search intent for professional audiences (investors, HCPs, talent) is well-aligned, but less so for top-of-funnel patient queries. The company maintains a consistent corporate presence across channels, though it is less focused on consumer-facing social media, which is appropriate for its model.
Exceptional content authority driven by a science-first narrative and in-depth information on its R&D engine, which builds immense credibility and attracts professional audiences.
Develop patient-centric, unbranded educational content hubs for key therapeutic areas to capture early-stage search traffic and better align with patient search intent.
Brand messaging is exceptionally clear and consistent, effectively positioning Regeneron as a science-driven innovator led by physician-scientists. The messaging is well-segmented for investors, partners, and potential employees, building strong credibility. However, the communication is highly rational and lacks a strong emotional connection, missing opportunities to showcase the human impact of its medicines through patient storytelling.
A powerful and unique brand narrative centered on 'homegrown,' repeatable innovation ('...over and over again'), which clearly differentiates it from competitors reliant on acquisitions.
Integrate compelling patient stories into the website to bridge the emotional gap, making the 'Science to Medicine' journey more tangible and resonant for a broader audience.
For its primary audiences (investors, talent, HCPs), the website offers a low-friction experience with clear navigation and a light cognitive load. The cross-device experience is excellent, ensuring accessibility on mobile platforms. 'Conversion' for these audiences (finding data, applying for jobs, locating trial info) is well-supported, but primary homepage CTAs could be more specific and action-oriented to better guide users.
Excellent mobile responsiveness and a clear, logical information architecture that allows professional users to find complex information efficiently and with minimal friction.
A/B test the main homepage call-to-action ('See what drives us') with more benefit-oriented and specific copy like 'Explore Our Pipeline' or 'Discover Our Science' to improve user guidance.
Regeneron projects world-class credibility through a robust hierarchy of trust signals, including its physician-scientist leadership, numerous industry awards, and a wealth of clinical data. The company's legal and compliance framework is exceptionally mature, demonstrating proactive risk mitigation, particularly around pharmaceutical marketing and data privacy. Third-party validation is consistently highlighted, reinforcing its position as an industry leader.
Exemplary legal and data privacy compliance, utilizing best-in-class platforms and demonstrating a sophisticated, proactive approach to mitigating regulatory risk globally.
Publish a formal, public-facing Accessibility Statement detailing its commitment to WCAG standards to formalize its dedication to digital inclusion and mitigate potential legal risks.
The company's competitive moat is deep and highly sustainable, built upon the proprietary VelociSuite® technologies and the massive data asset of the Regeneron Genetics Center (RGC). This creates a repeatable, efficient R&D engine that is exceptionally difficult for competitors to replicate. This 'homegrown' innovation model provides significant defensibility, although switching costs for its commercialized drugs are moderate and subject to competitive pressures.
The synergistic combination of the VelociSuite® platform and the Regeneron Genetics Center creates a durable, proprietary R&D engine that consistently generates novel drug targets and candidates.
Leverage the RGC data asset more explicitly in communications to position Regeneron as the leader in genetics-driven, personalized medicine, further widening the competitive gap.
Regeneron's business model is highly scalable, with high operating leverage on its blockbuster drugs and a deep pipeline of ~45 candidates poised for growth. The company is making significant investments to expand manufacturing capacity and has a proven track record of expanding products into new indications and global markets. Its strong financial position and strategic partnerships provide a robust foundation for future expansion.
A proven ability to execute label expansions for blockbuster drugs like Dupixent, consistently growing the addressable market and maximizing the value of its core assets.
Build out direct commercial capabilities in key international markets to retain more long-term value from its pipeline, reducing reliance on partners for global expansion.
Regeneron exhibits exceptional strategic focus, consistently allocating immense resources to its core R&D engine to drive long-term value. The business model is coherent and aligned with its science-first culture, leveraging partnerships effectively for commercialization. The primary weakness is a significant revenue concentration in two key products, which creates considerable risk despite the model's overall strength.
An unwavering strategic focus on its vertically integrated R&D engine, ensuring that resource allocation is consistently directed towards its primary source of competitive advantage.
Aggressively advance the late-stage oncology pipeline to establish a third major revenue pillar, reducing the critical business model risk of revenue concentration.
Regeneron holds significant market power and influence, demonstrated by its ability to establish blockbuster drugs like EYLEA and Dupixent as standards of care. The company has historically demonstrated strong pricing power, although this is now under pressure from competitors and biosimilars. While its market share is dominant in specific niches, it faces intense competition from larger pharmaceutical giants, making its market position a constant battle.
Sustained market leadership and influence in key therapeutic areas, built on a foundation of best-in-class clinical data and strong relationships with healthcare professionals.
Develop and deploy more sophisticated value-based pricing models to proactively defend formulary positions against new competitors and biosimilars, clearly demonstrating long-term cost-effectiveness to payers.
Business Overview
Business Classification
Biopharmaceutical
Research & Development (R&D) Intensive
Biotechnology
Sub Verticals
- •
Ophthalmology
- •
Immunology & Inflammation
- •
Oncology
- •
Genetic Medicines
- •
Cardiovascular & Metabolic Diseases
Mature
Maturity Indicators
- •
Over 35 years of operation
- •
Multiple blockbuster drugs with significant market share (e.g., Eylea, Dupixent).
- •
Consistent revenue and profitability.
- •
Extensive and advanced clinical pipeline with approximately 45 product candidates.
- •
Global commercial and operational footprint in over 100 countries.
- •
Established strategic partnerships with major pharmaceutical companies.
Enterprise
Steady
Revenue Model
Primary Revenue Streams
- Stream Name:
Net Product Sales
Description:Direct sales of proprietary, commercialized medicines, with Eylea and Libtayo being key wholly-owned products sold in the U.S.
Estimated Importance:Primary
Customer Segment:Specialty Distributors, Specialty Pharmacies, Hospitals, Physicians
Estimated Margin:High
- Stream Name:
Collaboration Revenue
Description:Represents Regeneron's share of profits from products co-developed and co-commercialized with strategic partners. This includes significant revenue from Dupixent (with Sanofi) and Eylea sales outside the U.S. (with Bayer).
Estimated Importance:Primary
Customer Segment:Strategic Partners (Sanofi, Bayer)
Estimated Margin:High
- Stream Name:
Technology Licensing & Royalties
Description:Fees and royalties from licensing proprietary technologies, such as the VelociSuite® platform, to other biopharmaceutical companies for their own drug discovery efforts.
Estimated Importance:Tertiary
Customer Segment:Other Pharmaceutical and Biotech Companies
Estimated Margin:Very High
Recurring Revenue Components
Treatments for chronic diseases (e.g., wet age-related macular degeneration, atopic dermatitis) requiring repeated administration.
Long-term collaboration and profit-sharing agreements.
Pricing Strategy
Value-Based Pricing
Premium
Opaque
Pricing Psychology
Focus on clinical efficacy and addressing high unmet medical needs to justify premium pricing.
Monetization Assessment
Strengths
- •
Strong revenue generation from blockbuster drugs Eylea and Dupixent.
- •
Diversified revenue through a mix of direct sales and profit-sharing from major collaborations.
- •
High-margin nature of proprietary biologic medicines.
Weaknesses
High revenue concentration in a few key products, creating significant risk from competition or patent expiry.
Dependence on partners for a substantial portion of revenue (e.g., Sanofi for Dupixent sales logistics).
Opportunities
- •
Successful launch and market penetration of Eylea HD to defend market share against biosimilars and competitors.
- •
Expansion of Dupixent into new indications, such as Chronic Obstructive Pulmonary Disease (COPD).
- •
Commercialization of late-stage pipeline candidates like Linvoseltamab to create new revenue streams.
Threats
- •
Imminent patent expirations for key products, particularly Eylea, opening the door for biosimilar competition.
- •
Increasing competition from established and emerging players (e.g., Roche's Vabysmo).
- •
Growing pricing pressure from governments, payers, and pharmacy benefit managers globally.
Market Positioning
A science-driven, innovation-led biopharmaceutical leader that repeatedly translates genetic insights and proprietary technologies into first-in-class or best-in-class medicines for serious diseases.
Market Leader in ophthalmology (anti-VEGF); Strong Contender in immunology and specific oncology niches.
Target Segments
- Segment Name:
Patients with Serious Diseases
Description:Individuals suffering from conditions with high unmet medical needs in Regeneron's core therapeutic areas: eye diseases, allergic/inflammatory diseases, cancer, cardiovascular diseases, and rare genetic disorders.
Demographic Factors
Varies by disease, but often includes aging populations (for eye diseases) or specific genetic markers.
Psychographic Factors
Seeking effective treatments to improve quality of life.
Highly motivated to manage chronic or life-threatening conditions.
Behavioral Factors
Adherence to prescribed treatment regimens.
Active participation in patient support programs.
Pain Points
- •
Lack of effective treatment options.
- •
Debilitating symptoms affecting daily life.
- •
High cost of specialty medicines.
Fit Assessment:Excellent
Segment Potential:High
- Segment Name:
Healthcare Professionals (HCPs)
Description:Specialist physicians who diagnose and treat patients in Regeneron's therapeutic areas, including ophthalmologists, dermatologists, allergists, oncologists, and cardiologists.
Demographic Factors
Board-certified specialists.
Practitioners in both hospital and private clinic settings.
Psychographic Factors
- •
Value clinical efficacy and safety data.
- •
Seek innovative treatments that offer clear advantages over standard of care.
- •
Influenced by peer-reviewed publications and key opinion leaders.
Behavioral Factors
Prescribe based on clinical trial data and treatment guidelines.
Attend medical conferences to stay updated on new therapies.
Pain Points
- •
Managing complex diseases with limited tools.
- •
Navigating insurance reimbursement and prior authorization processes.
- •
Patient non-adherence to complex treatment plans.
Fit Assessment:Excellent
Segment Potential:High
- Segment Name:
Payers and Health Systems
Description:Public and private insurance companies, government health programs (e.g., Medicare, Medicaid), and large hospital networks that manage drug formularies and reimbursement.
Demographic Factors
- •
National and regional health insurers.
- •
Government agencies.
- •
Integrated delivery networks (IDNs).
Psychographic Factors
Prioritize cost-effectiveness and budget impact.
Focus on value-based outcomes and evidence-based medicine.
Behavioral Factors
- •
Conduct pharmacoeconomic analyses.
- •
Negotiate rebates and discounts for formulary placement.
- •
Implement utilization management controls (e.g., prior authorization).
Pain Points
- •
Rising costs of specialty pharmaceuticals.
- •
Demonstrating long-term value and cost savings from new therapies.
- •
Managing large patient populations with chronic diseases.
Fit Assessment:Good
Segment Potential:Medium
Market Differentiation
- Factor:
Proprietary VelociSuite® Technologies
Strength:Strong
Sustainability:Sustainable
- Factor:
Regeneron Genetics Center (RGC)
Strength:Strong
Sustainability:Sustainable
- Factor:
Physician-Scientist Leadership and Science-First Culture
Strength:Moderate
Sustainability:Sustainable
- Factor:
Proven Track Record of 'Homegrown' Drug Development
Strength:Strong
Sustainability:Sustainable
Value Proposition
To use the power of science to repeatedly and consistently translate genetic insights and technological innovation into life-transforming medicines for people with serious diseases.
Excellent
Key Benefits
- Benefit:
Clinically Superior Treatments
Importance:Critical
Differentiation:Unique
Proof Elements
- •
FDA and global regulatory approvals for 14 medicines.
- •
Extensive positive Phase 3 clinical trial data.
- •
Market leadership of key products like Eylea and Dupixent.
- Benefit:
Innovation in Drug Discovery
Importance:Critical
Differentiation:Unique
Proof Elements
- •
Proprietary VelociSuite® technology platform enabling rapid antibody discovery.
- •
Regeneron Genetics Center's sequencing of ~3M exomes to identify novel drug targets.
- •
Robust pipeline of ~45 homegrown candidates.
- Benefit:
Addressing Unmet Medical Needs
Importance:Important
Differentiation:Somewhat unique
Proof Elements
Focus on rare diseases alongside more common conditions.
Development of medicines in challenging therapeutic areas like oncology and genetic diseases.
Unique Selling Points
- Usp:
The VelociSuite® technology platform, which enables industry-leading speed and efficiency in discovering and developing fully human antibodies.
Sustainability:Long-term
Defensibility:Strong
- Usp:
The Regeneron Genetics Center (RGC), a massive-scale human genetics research effort that provides a continuous source of validated, novel drug targets.
Sustainability:Long-term
Defensibility:Strong
- Usp:
An integrated, science-centric model where nearly all drug candidates are 'homegrown,' providing deep internal expertise and control from discovery through commercialization.
Sustainability:Long-term
Defensibility:Moderate
Customer Problems Solved
- Problem:
Vision loss due to chronic retinal diseases.
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Poorly controlled moderate-to-severe atopic dermatitis and asthma.
Severity:Major
Solution Effectiveness:Complete
- Problem:
Limited treatment options for certain advanced cancers.
Severity:Critical
Solution Effectiveness:Partial
Value Alignment Assessment
High
Regeneron's focus on high unmet need areas with aging populations and increasing prevalence of chronic diseases ensures strong, sustained demand for its innovative products.
High
The value proposition of superior clinical outcomes and novel mechanisms of action directly addresses the core needs of both patients seeking better quality of life and physicians seeking more effective treatment options.
Strategic Assessment
Business Model Canvas
Key Partners
- •
Sanofi (Co-development/commercialization of Dupixent, Kevzara, Praluent).
- •
Bayer (Co-development/commercialization of Eylea outside the U.S.).
- •
Roche (Past collaboration on REGEN-COV).
- •
Alnylam Pharmaceuticals (RNAi therapeutic collaboration).
- •
Intellia Therapeutics (CRISPR gene-editing research).
- •
Academic Institutions and Health Systems (For genetics research via RGC).
Key Activities
- •
Research & Development (Target identification, drug discovery, preclinical studies).
- •
Clinical Development (Managing global Phase I-III clinical trials).
- •
Biologics Manufacturing (Large-scale production of complex antibody-based medicines).
- •
Commercialization (Marketing, sales, and distribution).
- •
Regulatory Affairs (Navigating FDA, EMA, and other global health authority approvals).
Key Resources
- •
Intellectual Property (Extensive patent portfolio for products and technologies).
- •
Proprietary Technology Platforms (VelociSuite®, Regeneron Genetics Center).
- •
Scientific and Clinical Talent (High concentration of MDs and PhDs).
- •
State-of-the-Art R&D and Manufacturing Facilities.
- •
Strong Financial Position (Capital for R&D investment and business development).
Cost Structure
- •
High Research & Development (R&D) expenses (Invested $4.4 billion in 2023).
- •
Sales, General & Administrative (SG&A) costs for commercial products.
- •
Cost of Goods Sold (COGS) related to manufacturing.
- •
Costs associated with clinical trials and regulatory submissions.
Swot Analysis
Strengths
- •
World-class, vertically integrated R&D engine with a track record of consistent innovation.
- •
Portfolio of blockbuster drugs providing strong cash flow for reinvestment.
- •
Defensible competitive moat built on proprietary technologies (VelociSuite®, RGC).
- •
Strategic, value-accretive partnerships with global pharmaceutical leaders.
Weaknesses
- •
Significant revenue concentration on two key assets (Eylea and Dupixent).
- •
Dependence on partners for ex-U.S. commercialization, limiting direct global market control.
- •
Limited experience in small molecule drugs, focusing almost exclusively on biologics and genetic medicines.
Opportunities
- •
Leveraging the RGC database to accelerate entry into genetic medicines and personalized health.
- •
Successfully converting the market from Eylea to next-generation Eylea HD to extend franchise lifecycle.
- •
Expanding the oncology portfolio with late-stage assets to build a third major therapeutic pillar.
- •
Strategic acquisitions to in-license late-stage assets or new technology platforms.
Threats
- •
Looming patent cliffs and the market entry of biosimilar versions of Eylea.
- •
Intensifying competition from novel therapies in core areas (e.g., Roche's Vabysmo in ophthalmology).
- •
Global downward pressure on drug pricing and reimbursement from payers and governments.
- •
The inherent risk of clinical trial failures for high-potential pipeline candidates.
Recommendations
Priority Improvements
- Area:
Revenue Diversification
Recommendation:Aggressively advance and invest in the late-stage oncology pipeline (e.g., Linvoseltamab) to establish a third major revenue pillar and reduce reliance on Eylea and Dupixent.
Expected Impact:High
- Area:
Lifecycle Management
Recommendation:Execute a flawless commercial strategy for Eylea HD, emphasizing its clinical benefits (extended dosing intervals) to maximize patient and physician conversion ahead of biosimilar entry.
Expected Impact:High
- Area:
Market Access Strategy
Recommendation:Proactively develop and deploy value-based pricing and access models to defend formulary positions against new competitors and biosimilars, demonstrating long-term cost-effectiveness to payers.
Expected Impact:Medium
Business Model Innovation
Monetize the Regeneron Genetics Center data through strategic partnerships with diagnostic companies or digital health platforms to create predictive and personalized medicine solutions.
Establish a dedicated internal venture arm to invest in and acquire emerging biotech companies with complementary technologies, particularly in areas beyond antibodies (e.g., cell therapy, RNA therapeutics).
Revenue Diversification
Pursue targeted M&A to acquire late-stage or commercial assets in adjacent therapeutic areas to leverage existing commercial infrastructure.
Expand ex-U.S. commercial presence by selectively renegotiating partnership terms or building direct commercial capabilities in key international markets.
Regeneron's business model is a paradigm of a science-driven, vertically integrated biopharmaceutical enterprise. Its core strength and competitive advantage are deeply rooted in its unparalleled R&D engine, powered by the proprietary VelociSuite® and Regeneron Genetics Center platforms. This has enabled a repeatable process of 'homegrown' innovation, leading to blockbuster products like Eylea and Dupixent that generate substantial cash flow. The model's primary strategic challenge is its significant revenue concentration, making it vulnerable to patent expirations and intensifying competition for its key assets. Future success is contingent on executing a critical strategic transition: successfully defending its ophthalmology franchise through lifecycle management (Eylea HD), while simultaneously leveraging its R&D prowess to commercialize its deep pipeline, particularly in oncology, to achieve necessary revenue diversification. The company's ability to evolve from a two-product success story into a multi-pillar, global biopharmaceutical leader will define its next chapter. Strategic evolution should focus on monetizing its vast genetic data assets in new ways and potentially using its strong financial position for targeted M&A to accelerate diversification beyond its current therapeutic strongholds.
Competitors
Competitive Landscape
Growth/Mature
Moderately concentrated
Barriers To Entry
- Barrier:
High R&D and Clinical Trial Costs
Impact:High
- Barrier:
Stringent and Lengthy Regulatory Approval Processes (FDA, EMA)
Impact:High
- Barrier:
Intellectual Property (Patents)
Impact:High
- Barrier:
Complex and Specialized Manufacturing Capabilities
Impact:High
- Barrier:
Established Commercialization and Distribution Networks
Impact:Medium
Industry Trends
- Trend:
Personalized/Precision Medicine and Genetic Diagnostics
Impact On Business:Major opportunity for Regeneron given its investment in the Regeneron Genetics Center (RGC), enabling novel target identification.
Timeline:Immediate
- Trend:
AI/ML in Drug Discovery and Development
Impact On Business:Opportunity to accelerate R&D timelines and improve success rates by integrating AI with their vast genetic and clinical data.
Timeline:Near-term
- Trend:
Rise of Gene and Cell Therapies
Impact On Business:A strategic growth area that requires new capabilities but aligns with Regeneron's focus on genetic medicines.
Timeline:Near-term
- Trend:
Increased Competition from Biosimilars
Impact On Business:Significant threat to revenue streams of blockbuster biologics like EYLEA as patents expire.
Timeline:Immediate
- Trend:
Global Drug Pricing Pressure and Regulatory Scrutiny
Impact On Business:Potential for margin compression and need for demonstrating clear value and cost-effectiveness for new and existing therapies.
Timeline:Immediate
Direct Competitors
- →
Amgen Inc.
Market Share Estimate:Varies by therapeutic area
Target Audience Overlap:High
Competitive Positioning:A leading biotechnology pioneer with a strong portfolio in inflammation, oncology, and cardiovascular disease.
Strengths
- •
Broad and diversified portfolio of blockbuster drugs.
- •
Strong global commercial presence and distribution network.
- •
Significant investment in R&D and a robust pipeline.
- •
Expertise in both biologics and small molecules.
Weaknesses
- •
Facing patent expirations on key products.
- •
Dependency on a few key drugs for a large portion of revenue.
- •
Increasing competition from biosimilars for its established products.
Differentiators
- •
Direct competitor to Praluent with their PCSK9 inhibitor, Repatha.
- •
Leader in bone health and nephrology, areas where Regeneron is less focused.
- •
Strong focus on acquiring external innovation to supplement internal R&D.
- →
Genentech (a subsidiary of Roche)
Market Share Estimate:Varies by therapeutic area
Target Audience Overlap:High
Competitive Positioning:A science-driven leader in oncology, immunology, and ophthalmology with a history of breakthrough innovations.
Strengths
- •
Dominant position in the oncology market with numerous successful therapies.
- •
Strong R&D capabilities and a deep pipeline.
- •
Extensive global resources and marketing power through Roche.
- •
Leader in personalized healthcare and diagnostics.
Weaknesses
- •
Facing biosimilar competition for some of its older, highly successful biologics (e.g., Rituxan, Herceptin).
- •
Complex organizational structure as part of the larger Roche group.
- •
High-profile patent disputes.
Differentiators
- •
Direct and formidable competitor in ophthalmology with products like Vabysmo and Lucentis, challenging EYLEA's market share.
- •
Pioneer in antibody-drug conjugates (ADCs) and bispecific antibodies.
- •
Integration with Roche's diagnostic capabilities enables a strong personalized medicine approach.
- →
Novartis AG
Market Share Estimate:Varies by therapeutic area
Target Audience Overlap:Medium-High
Competitive Positioning:A global healthcare company with a diversified portfolio across innovative medicines, including pharmaceuticals, gene therapies, and a strong focus on R&D.
Strengths
- •
Highly diversified portfolio across multiple therapeutic areas.
- •
Leader in cell and gene therapies (e.g., Zolgensma).
- •
Strong global presence, particularly in emerging markets.
- •
Significant annual investment in R&D.
Weaknesses
- •
Facing challenges from patent expirations and generic competition.
- •
Has undergone significant restructuring, which can create internal disruption.
- •
Past legal and ethical controversies have impacted brand reputation.
Differentiators
- •
Direct competitor in ophthalmology (Lucentis) and immunology (Cosentyx).
- •
Stronger focus on advanced therapy modalities like cell therapy and radioligand therapy.
- •
Competes with Praluent via its cholesterol-lowering drug, Leqvio.
- →
Sanofi
Market Share Estimate:Varies by therapeutic area
Target Audience Overlap:High
Competitive Positioning:A global biopharmaceutical company focused on human health, with strongholds in immunology, vaccines, and rare diseases.
Strengths
- •
Strong and successful commercial partnership with Regeneron on key drugs like Dupixent and Praluent.
- •
Leading global position in vaccines.
- •
Diversified portfolio across pharmaceuticals, vaccines, and consumer healthcare.
- •
Strong presence in emerging markets.
Weaknesses
- •
Historically faced pipeline challenges and reliance on older blockbuster drugs.
- •
Revenue concentration risk, with heavy reliance on the success of Dupixent.
- •
Navigating a strategic shift away from some traditional areas like diabetes.
Differentiators
- •
A unique 'co-petitor' relationship; they are Regeneron's most important collaborator but also a potential competitor in other areas.
- •
Deep expertise in vaccine development and manufacturing.
- •
Strong focus on rare diseases through its Genzyme division.
Indirect Competitors
- →
Specialized Gene-Editing Startups (e.g., Intellia Therapeutics, CRISPR Therapeutics)
Description:Companies pioneering novel therapeutic modalities like CRISPR-based gene editing to offer potential one-time cures for genetic diseases.
Threat Level:Medium
Potential For Direct Competition:High, especially as their platforms mature and they target diseases in Regeneron's pipeline.
- →
AI-driven Drug Discovery Companies (e.g., Recursion, Exscientia)
Description:Tech-bio companies using artificial intelligence and machine learning to dramatically accelerate the identification of novel drug targets and candidates, potentially disrupting the traditional R&D model.
Threat Level:Low-Medium
Potential For Direct Competition:Medium, more likely to become partners but could enable new, faster-moving competitors.
- →
Big Tech (e.g., Google/Verily, NVIDIA)
Description:Large technology companies entering the healthcare space with massive data processing capabilities, AI platforms, and a focus on diagnostics, genomics, and clinical trial optimization.
Threat Level:Low
Potential For Direct Competition:Low in the short-term, but high potential for disruption in the R&D and data analysis landscape.
Competitive Advantage Analysis
Sustainable Advantages
- Advantage:
Proprietary
VelociSuite®Technology PlatformSustainability Assessment:Highly sustainable. This integrated suite of technologies for antibody discovery and development provides a consistent, rapid, and efficient engine for generating homegrown drug candidates.
Competitor Replication Difficulty:Hard
- Advantage:
Regeneron Genetics Center (RGC)
Sustainability Assessment:Highly sustainable. The large-scale human genetics sequencing effort provides a unique and powerful data asset for identifying novel, validated drug targets, creating a durable R&D advantage.
Competitor Replication Difficulty:Hard
- Advantage:
Science-First, Physician-Scientist-Led Culture
Sustainability Assessment:Sustainable. The company's founding principle of being led by science and scientists fosters a culture of relentless innovation and long-term R&D focus that is difficult to replicate.
Competitor Replication Difficulty:Hard
Temporary Advantages
{'advantage': 'Market Dominance of EYLEA', 'estimated_duration': '1-3 years'}
{'advantage': 'Exclusivity on Dupixent (with Sanofi)', 'estimated_duration': '3-5 years'}
Disadvantages
- Disadvantage:
Revenue Concentration
Impact:Critical
Addressability:Moderately
- Disadvantage:
Looming Biosimilar Competition
Impact:Major
Addressability:Difficult
- Disadvantage:
Dependence on Key Partnerships (notably Sanofi)
Impact:Major
Addressability:Difficult
Strategic Recommendations
Quick Wins
- Recommendation:
Amplify the 'Homegrown Innovation' narrative in corporate communications to differentiate from competitors reliant on M&A.
Expected Impact:Medium
Implementation Difficulty:Easy
- Recommendation:
Launch a targeted digital campaign showcasing the power of the RGC in finding novel drug targets to reinforce the image of a futuristic, data-driven biotech.
Expected Impact:Medium
Implementation Difficulty:Moderate
Medium Term Strategies
- Recommendation:
Aggressively advance the oncology pipeline, particularly bispecific antibodies, to diversify revenue away from EYLEA and Dupixent.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Secure strategic 'bolt-on' acquisitions or licensing deals for complementary technologies, such as novel drug delivery systems or AI platforms, to enhance the VelociSuite® capabilities.
Expected Impact:High
Implementation Difficulty:Moderate
- Recommendation:
Expand the global commercial footprint independently of partners in key markets to retain more long-term value from homegrown assets.
Expected Impact:High
Implementation Difficulty:Difficult
Long Term Strategies
- Recommendation:
Fully operationalize the RGC as a predictive engine for personalized medicine, developing companion diagnostics alongside new therapeutics.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Invest in building internal expertise and manufacturing capabilities for next-generation therapeutic modalities like mRNA or in-vivo gene editing.
Expected Impact:High
Implementation Difficulty:Difficult
Solidify and promote the position as the most consistently innovative, science-driven biopharma that repeatedly translates fundamental genetic insights into homegrown, life-changing medicines.
Differentiate based on the 'repeatable and predictable' nature of the R&D engine (VelociSuite + RGC), in contrast to competitors who are more reliant on one-off blockbusters or M&A. Emphasize the speed, efficiency, and quality of the internal discovery-to-development pipeline.
Whitespace Opportunities
- Opportunity:
Develop AI/ML platforms that integrate genomic data (from RGC) with clinical and real-world data to predict patient response and identify novel biomarkers.
Competitive Gap:While many competitors use AI, few have the proprietary, large-scale, integrated genomic data asset that Regeneron possesses.
Feasibility:Medium
Potential Impact:High
- Opportunity:
Pioneer treatments for 'undiagnosed' rare genetic diseases identified through the RGC, creating entirely new markets where Regeneron is the first and only player.
Competitive Gap:Most competitors target known rare diseases; the RGC allows for the discovery of new ones, creating a first-mover advantage.
Feasibility:Medium
Potential Impact:High
- Opportunity:
Expand into digital therapeutics (DTx) that complement key franchises (e.g., an app to help manage atopic dermatitis for Dupixent patients), creating a stickier ecosystem and capturing valuable patient data.
Competitive Gap:Many large pharma companies are still nascent in their integration of DTx with their core therapeutic products.
Feasibility:High
Potential Impact:Medium
Regeneron has established itself as a premier, science-driven biotechnology firm, built upon the formidable and difficult-to-replicate competitive advantages of its VelociSuite® technology platform and the Regeneron Genetics Center (RGC). This integrated, homegrown R&D engine allows the company to consistently and efficiently translate genetic insights into novel therapeutics, a key differentiator in an industry often reliant on M&A for pipeline growth. The company's primary focus areas are in ophthalmology, allergic and inflammatory diseases, and oncology.
The competitive landscape is intense, populated by large, well-funded biopharmaceutical giants like Amgen, Novartis, and the Roche/Genentech entity. Direct competition is particularly fierce in key therapeutic areas. In ophthalmology, EYLEA faces significant pressure from Roche/Genentech's Vabysmo. In immunology, Dupixent competes in a crowded market, and in the cardiovascular space, Praluent contends with Amgen's Repatha. These competitors possess diversified portfolios, extensive global commercial reach, and massive R&D budgets, representing a constant threat to Regeneron's market share.
Regeneron's most significant vulnerability is its heavy revenue concentration on two blockbuster products, EYLEA and Dupixent (in partnership with Sanofi), making it susceptible to patent cliffs and biosimilar competition. The strategic imperative is clear: leverage the proven strength of its R&D engine to aggressively diversify its pipeline and commercial portfolio, particularly in high-growth areas like oncology.
The primary strategic opportunity lies in fully weaponizing the RGC data asset. By integrating advanced AI and machine learning, Regeneron can accelerate target identification and create a new generation of precision medicines, potentially for rare diseases that competitors have not yet identified. This would allow Regeneron to move from competing in crowded markets to creating new ones. To secure long-term success, Regeneron must successfully transition from a company defined by a few key blockbusters to one recognized for its unparalleled, repeatable innovation platform that consistently delivers the next wave of transformative medicines.
Messaging
Message Architecture
Key Messages
- Message:
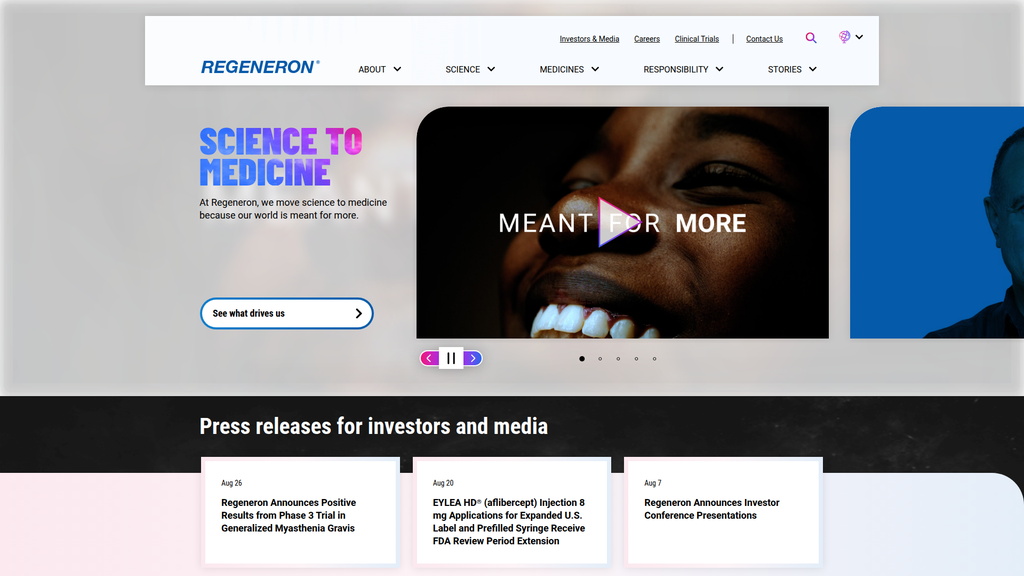
SCIENCE TO MEDICINE
Prominence:Primary
Clarity Score:High
Location:Homepage Hero Banner
- Message:
Our mission is to use the power of science to bring new medicines to patients ... over and over again.
Prominence:Primary
Clarity Score:High
Location:About Us Page
- Message:
Pioneering a new era in biopharmaceutical innovation
Prominence:Secondary
Clarity Score:High
Location:Homepage
- Message:
We're guided by our philosophy of doing well by doing good.
Prominence:Secondary
Clarity Score:High
Location:Homepage
- Message:
Supporting future STEM talent.
Prominence:Tertiary
Clarity Score:Medium
Location:Homepage
The messaging hierarchy is logical and effective. It starts with the core purpose ('SCIENCE TO MEDICINE'), expands to the 'how' (innovation and R&D), and then covers the 'why' (societal impact, responsibility, and STEM support). This structure clearly communicates the company's identity and values from a high level down to supporting initiatives.
Messaging is highly consistent across the analyzed pages. The core theme of translating science into medicine is reinforced on both the homepage and the 'About Us' page, creating a unified and memorable brand narrative.
Brand Voice
Voice Attributes
- Attribute:
Authoritative & Expert-Driven
Strength:Strong
Examples
- •
Founded and led by physician-scientists...
- •
EYE ON THE EXPERTS
- •
...our leadership team includes Nobel Laureates and eight members of the National Academy of Sciences...
- Attribute:
Innovative & Pioneering
Strength:Strong
Examples
- •
George’s Five Principles for Radical Innovation
- •
Pioneering a new era in biopharmaceutical innovation
- •
Regeneron pushes the boundaries of scientific discovery...
- Attribute:
Purpose-Driven & Aspirational
Strength:Moderate
Examples
- •
...our world is meant for more.
- •
...doing well by doing good
- •
Our journey of discovery continues as we strive to transform the lives of people with serious diseases.
- Attribute:
Confident & Established
Strength:Strong
Examples
- •
We are a leading biotechnology company...
- •
...our unique ability to repeatedly and consistently translate science into medicine...
- •
35+ years of scientific leadership
Tone Analysis
Professional
Secondary Tones
- •
Scientific
- •
Aspirational
- •
Corporate
Tone Shifts
The tone shifts to be more formal and legalistic on the 'Privacy Notice' page, which is appropriate and expected for that type of content.
Voice Consistency Rating
Excellent
Consistency Issues
No itemsValue Proposition Assessment
Regeneron is a leading, science-first biotechnology company, founded and run by physician-scientists, that leverages proprietary technologies to consistently invent, develop, and commercialize homegrown, life-transforming medicines.
Value Proposition Components
- Component:
Physician-Scientist Leadership
Clarity:Clear
Uniqueness:Unique
- Component:
Repeatable Innovation Engine ('...over and over again')
Clarity:Clear
Uniqueness:Unique
- Component:
Homegrown Pipeline (~100% in-house)
Clarity:Clear
Uniqueness:Somewhat Unique
- Component:
Proprietary Technology Platforms (VelociSuite®, Regeneron Genetics Center®)
Clarity:Clear
Uniqueness:Unique
- Component:
Commitment to Corporate Responsibility and STEM
Clarity:Clear
Uniqueness:Common
Regeneron's messaging effectively differentiates the company from competitors. The emphasis on being 'founded and led by physician-scientists' builds significant credibility. The narrative of a homegrown, repeatable innovation process ('over and over again') powerfully contrasts with competitors who may rely more heavily on acquisitions. This positions Regeneron not just as a drugmaker, but as a fundamental discovery engine.
The messaging positions Regeneron as a pure, science-driven innovator at the top of the biotech industry. It suggests a culture of deep scientific inquiry rather than a primary focus on sales and marketing, which may appeal to top scientific talent, research partners, and long-term investors. It competes with giants like Amgen and Roche by highlighting its unique, integrated R&D model.
Audience Messaging
Target Personas
- Persona:
Investors & Financial Analysts
Tailored Messages
- •
Press releases for investors and media
- •
Regeneron at a glance (stats on pipeline, approved medicines, revenue)
- •
View our annual materials
Effectiveness:Effective
- Persona:
Scientific Talent & Job Seekers
Tailored Messages
- •
Join the brightest, most passionate innovators in science.
- •
Top 5 ranking in Science magazine's global Top Employer survey...
- •
Founded and led by physician-scientists
- •
Explore our careers
Effectiveness:Effective
- Persona:
Patients & Caregivers
Tailored Messages
We aim to push the bounds of science to make LIFE-CHANGING MEDICINES
Discover more about our clinical trials or locate a relevant trial near you.
Effectiveness:Somewhat Effective
- Persona:
Potential Business/Research Partners
Tailored Messages
We take a highly collaborative approach to science and drug development...
How to partner with Regeneron
Effectiveness:Effective
Audience Pain Points Addressed
The societal and personal burden of serious diseases (e.g., eye diseases, cancer, inflammatory diseases).
The challenge and high failure rate of traditional drug discovery.
Audience Aspirations Addressed
- •
A healthier future with transformative treatments for serious illnesses ('our world is meant for more').
- •
Advancing human knowledge through scientific discovery.
- •
A desire for corporations to act responsibly and invest in the future (STEM education).
Persuasion Elements
Emotional Appeals
- Appeal Type:
Hope
Effectiveness:Medium
Examples
LIFE-CHANGING MEDICINES
...strive to transform the lives of people with serious diseases.
- Appeal Type:
Pride & Prestige
Effectiveness:High
Examples
- •
Honors and recognitions section (Dow Jones, Civic 50, etc.)
- •
Top 5 ranking in Science magazine's global Top Employer survey...
- •
Nobel Laureates and eight members of the National Academy of Sciences...
Social Proof Elements
- Proof Type:
Expert Endorsement (Leadership Credentials)
Impact:Strong
- Proof Type:
Awards and Rankings
Impact:Strong
- Proof Type:
Data & Statistics ('Regeneron at a glance')
Impact:Strong
Trust Indicators
- •
Leadership by 'physician-scientists'
- •
Prominently displayed press releases with clinical trial data
- •
List of peer-reviewed publications
- •
35+ year company history
Scarcity Urgency Tactics
No itemsCalls To Action
Primary Ctas
- Text:
See what drives us
Location:Homepage Hero
Clarity:Clear
- Text:
Join our team
Location:Homepage
Clarity:Clear
- Text:
Explore our clinical trials
Location:Homepage
Clarity:Clear
- Text:
Learn more about our research and development efforts
Location:Homepage
Clarity:Clear
The CTAs are clear, well-placed, and effectively segmented for different audiences. They guide users into distinct funnels (Careers, R&D, Clinical Trials, Corporate Info) rather than pushing for a single, universal action. This approach is highly appropriate for a corporate website with diverse visitor goals.
Messaging Gaps Analysis
Critical Gaps
The website lacks a strong, direct patient-centric narrative. While the mission is patient-focused, the content primarily celebrates the science and the company. The human impact of the 'life-changing medicines' is stated but not shown through storytelling.
Contradiction Points
No itemsUnderdeveloped Areas
Storytelling: The narrative is very corporate and scientific. Integrating patient stories or 'day-in-the-life' stories of their scientists could add a powerful emotional layer and make the 'Science to Medicine' journey more tangible for a broader audience.
Messaging Quality
Strengths
- •
Extremely clear, consistent, and memorable core message ('Science to Medicine').
- •
Powerful and unique differentiation through its 'physician-scientist led' and 'homegrown' innovation narrative.
- •
Excellent use of social proof and trust indicators to build authority and credibility.
- •
The brand voice is authoritative and confident without being arrogant.
Weaknesses
The messaging is highly rational and can feel emotionally distant.
The direct connection between the company's scientific process and the end benefit for a patient's life is not vividly illustrated.
Opportunities
Elevate the concept of 'over and over again' from the mission statement into a more prominent marketing message to underscore their unique capability for sustained innovation.
Develop a content stream dedicated to patient stories (anonymized or with consent) to bridge the emotional gap and humanize the brand.
Optimization Roadmap
Priority Improvements
- Area:
Patient-Centric Storytelling
Recommendation:Introduce a new content section on the homepage and within the 'About' section titled 'The Human Impact of Our Science'. This section should feature compelling, sensitive, and authentic stories that illustrate how Regeneron's medicines have transformed patient lives. Use a mix of short videos, articles, and infographics.
Expected Impact:High
- Area:
Value Proposition Reinforcement
Recommendation:Create a dedicated, visually engaging landing page explaining the 'Regeneron Way' of innovation. This should detail the proprietary technologies (VelociSuite®, RGC) and the unique 'homegrown' R&D philosophy, making this key differentiator more accessible and understandable.
Expected Impact:Medium
Quick Wins
Integrate the powerful phrase '...over and over again' into key headlines and sub-headlines on the homepage and 'About Us' page to reinforce the message of repeatable success.
Add more photos and videos of Regeneron's actual scientists and labs in action to make the 'science' component of the brand message more tangible and human.
Long Term Recommendations
Develop tailored content hubs for key audiences (HCPs, Patients, Investors) accessible from the main navigation. Each hub should feature messaging, resources, and user paths specifically designed for that persona's needs and journey.
Invest in thought leadership content that goes beyond the company's own work, exploring the future of biotechnology and medicine to solidify Regeneron's position as an industry-leading voice.
Regeneron's strategic messaging is world-class in its clarity, consistency, and differentiation. The core narrative of 'Science to Medicine,' driven by a unique 'physician-scientist led' and 'homegrown' innovation engine, effectively positions the company as a credible, authoritative leader in the biotechnology industry. The messaging architecture is logical, guiding distinct audiences like investors, partners, and potential talent to relevant information with clear calls-to-action. The brand voice is consistently professional, confident, and innovative, supported by a wealth of social proof, including prestigious awards and impressive company statistics.
The primary opportunity for enhancement lies in bridging the emotional gap between the company's process-focused narrative and the ultimate human impact of its work. The current messaging excels at explaining the 'what' and 'how' of Regeneron's business but is less effective at 'showing' the 'why' from a patient's perspective. While the mission is clearly patient-centric, the website's content is heavily weighted toward corporate achievements, scientific principles, and R&D processes. By integrating more compelling patient-centric storytelling, Regeneron can add a powerful emotional dimension to its already strong rational appeal. This would not only humanize the brand for a broader audience but also make the value of its scientific innovation more tangible and resonant, thereby strengthening its market positioning and overall brand equity.
Growth Readiness
Growth Foundation
Product Market Fit
Strong
Evidence
- •
Blockbuster status of key drugs: EYLEA for eye diseases and Dupixent (with Sanofi) for inflammatory conditions are multi-billion dollar products, indicating profound market acceptance and physician trust.
- •
Sustained revenue generation: Despite competition, EYLEA and Dupixent continue to drive significant revenue, with Dupixent sales growing 22.8% in Q1 2025.
- •
Label expansion success: Dupixent's continued approval for new indications (e.g., atopic dermatitis, asthma, COPD) demonstrates its versatility and strong clinical value, consistently expanding its addressable market.
- •
Robust R&D Engine: The company's mission to 'use the power of science to bring new medicines to patients... over and over again' is validated by its ~45 product candidates in development, almost all homegrown.
Improvement Areas
- •
Diversify revenue beyond EYLEA and Dupixent to mitigate concentration risk.
- •
Accelerate commercialization of late-stage pipeline assets, particularly in oncology and rare diseases, to establish new pillars of growth.
- •
Strengthen the value proposition of EYLEA HD to effectively compete with biosimilars and new market entrants like Roche's Vabysmo.
Market Dynamics
Consistently high, with the global biotechnology market projected to grow at a CAGR of 12-13.6% through 2032-2034.
Mature
Market Trends
- Trend:
Rise of AI in Drug Discovery
Business Impact:Opportunity to accelerate R&D timelines and increase discovery precision by leveraging its massive dataset from the Regeneron Genetics Center. Requires investment in computational biology and AI partnerships.
- Trend:
Growth in Gene & Cell Therapies
Business Impact:Significant growth vector. The gene therapy market is projected to grow at a CAGR of ~19-33%. Regeneron's investments in this area are crucial for long-term leadership.
- Trend:
Patent Cliffs and Biosimilar Competition
Business Impact:Major threat to key revenue streams, especially for EYLEA, with key patents expiring. This necessitates a robust lifecycle management strategy and a strong pipeline to offset revenue erosion.
- Trend:
Increased Focus on Personalized Medicine
Business Impact:Strengthens Regeneron's genetics-first approach. The Regeneron Genetics Center provides a powerful platform to identify patient subgroups and develop targeted therapies, a key competitive advantage.
Excellent. The biotechnology sector is in a high-growth phase, with significant innovation in areas where Regeneron has core strengths (biologics, genetics). However, the timing is also critical due to the imminent patent cliff for EYLEA, making the next 2-3 years pivotal for launching new products.
Business Model Scalability
High
High fixed costs associated with R&D and biologics manufacturing facilities, but low variable costs per unit once a drug is established, leading to high operating leverage on successful products.
High. The business model is built on creating blockbuster drugs where revenue far outstrips the incremental cost of production and sales, leading to substantial profit margins on mature products.
Scalability Constraints
- •
Complex and capital-intensive manufacturing processes for biologics can be a bottleneck for rapid scaling of new products.
- •
Navigating global regulatory approvals and reimbursement negotiations for each new drug or indication is time-consuming and complex.
- •
Reliance on strategic partners (e.g., Sanofi, Bayer) for co-commercialization in certain areas can introduce dependencies.
Team Readiness
Exceptional. Founded and still led by physician-scientists, with a deep-rooted, science-first culture. The leadership team has a proven track record of translating scientific discovery into commercial success.
Effective. The structure supports a powerful, centralized R&D engine with business development and commercial teams built around key therapeutic areas. Highly collaborative model evidenced by numerous strategic partnerships.
Key Capability Gaps
- •
Scaling expertise in gene therapy manufacturing and supply chain logistics.
- •
Expanded talent in computational biology, data science, and AI/ML to fully leverage the Regeneron Genetics Center data.
- •
Strengthening commercial presence and market access teams in emerging global markets, particularly in Asia-Pacific.
Growth Engine
Acquisition Channels
- Channel:
Key Opinion Leader (KOL) Engagement & Scientific Publications
Effectiveness:High
Optimization Potential:Medium
Recommendation:Increase digital engagement with KOLs through virtual advisory boards and leverage data analytics to identify rising influencers in new therapeutic areas like gene therapy.
- Channel:
Specialty Sales Force & Medical Science Liaisons (MSLs)
Effectiveness:High
Optimization Potential:High
Recommendation:Equip sales and MSL teams with advanced analytics and real-world evidence (RWE) platforms to deliver personalized, data-driven insights to healthcare providers (HCPs) and payers.
- Channel:
Payer & Formulary Access Teams
Effectiveness:High
Optimization Potential:Medium
Recommendation:Develop more sophisticated value-based pricing and contracting models, especially for high-cost gene therapies, to ensure favorable formulary placement and patient access.
- Channel:
Direct-to-Consumer (DTC) Advertising (for specific products like Dupixent)
Effectiveness:Medium
Optimization Potential:High
Recommendation:Shift DTC spend towards targeted digital channels and patient advocacy group partnerships to improve ROI and reach highly motivated patient populations.
Customer Journey
The 'customer' journey involves multiple stakeholders: Patient (symptom awareness to adherence), Physician (diagnosis to prescription), and Payer (coverage determination). The path is complex and heavily mediated by clinical data and reimbursement.
Friction Points
- •
Complex reimbursement and prior authorization processes for specialty drugs, creating delays in treatment initiation.
- •
Patient adherence challenges for chronic conditions requiring self-injection or regular infusions.
- •
Competition from biosimilars and alternative therapies influencing physician prescribing habits.
Journey Enhancement Priorities
{'area': 'Patient Support Programs', 'recommendation': 'Invest in digital health tools (e.g., adherence apps, telehealth support) integrated with patient support services to simplify the patient experience and improve long-term adherence.'}
{'area': 'Physician Education', 'recommendation': 'Develop a robust digital ecosystem for HCPs providing on-demand access to clinical data, RWE, and peer-to-peer educational content for new and existing therapies.'}
Retention Mechanisms
- Mechanism:
Positive Long-Term Clinical Data & Real-World Evidence
Effectiveness:High
Improvement Opportunity:Proactively generate and publish RWE studies that demonstrate long-term value, safety, and cost-effectiveness to reinforce physician and payer confidence.
- Mechanism:
Patient Support and Adherence Programs
Effectiveness:Medium
Improvement Opportunity:Personalize support programs using data analytics to identify patients at risk of non-adherence and provide targeted interventions.
- Mechanism:
Lifecycle Management (e.g., EYLEA HD)
Effectiveness:High
Improvement Opportunity:Ensure seamless transition for patients and physicians to next-generation products by clearly articulating the clinical benefits (e.g., extended dosing intervals) and securing broad payer coverage.
Revenue Economics
Extremely Favorable. For blockbuster drugs, the high-margin revenue per patient far exceeds the variable costs of manufacturing and commercialization, after accounting for massive upfront R&D investment. Patent protection is the key determinant of profitability duration.
Not Applicable. A more relevant metric is 'Peak Sales Potential vs. R&D and Commercialization Cost', which for drugs like Dupixent and EyleA is exceptionally high.
High
Optimization Recommendations
- •
Maximize the duration and revenue of existing blockbusters through strategic label expansions into new patient populations.
- •
Improve R&D efficiency by using the genetics platform to increase the probability of success for clinical candidates, thereby lowering the average cost per approved drug.
- •
Optimize partnership structures to share R&D costs and commercialization expenses, enhancing the risk-adjusted return on investment (e.g., Sanofi collaboration).
Scale Barriers
Technical Limitations
- Limitation:
Biologics Manufacturing Capacity
Impact:High
Solution Approach:Continued investment in expanding in-house manufacturing capabilities and securing strategic partnerships with contract development and manufacturing organizations (CDMOs) to ensure supply chain redundancy and flexibility for new product launches.
- Limitation:
Complexity of Gene Therapy Development
Impact:High
Solution Approach:Invest heavily in proprietary vector delivery technologies and manufacturing processes. Consider strategic acquisitions of smaller biotechs with specialized gene therapy platforms or manufacturing expertise.
Operational Bottlenecks
- Bottleneck:
Global Clinical Trial Recruitment
Growth Impact:Can delay pipeline progression and time-to-market for new drugs.
Resolution Strategy:Utilize AI and RWE to optimize trial site selection and patient identification. Implement decentralized clinical trial (DCT) models to broaden patient access and accelerate enrollment.
- Bottleneck:
International Regulatory & Reimbursement Timelines
Growth Impact:Staggered global launches can delay peak sales realization and allow competitors to gain a foothold in key markets.
Resolution Strategy:Build out dedicated regional market access teams and engage with regulatory bodies and payers earlier in the development process to align on evidence requirements and streamline approvals.
Market Penetration Challenges
- Challenge:
Patent Cliff and Biosimilar Entry for EYLEA
Severity:Critical
Mitigation Strategy:Execute a flawless commercial strategy for EYLEA HD to switch existing patients. Aggressively defend valid patents while accelerating pipeline assets in ophthalmology to offer a next-generation alternative.
- Challenge:
Intense Competition in Crowded Therapeutic Areas (e.g., Oncology, Immunology)
Severity:Major
Mitigation Strategy:Focus on developing drugs with novel mechanisms of action or best-in-class profiles. Utilize the genetics platform to identify unique targets and patient populations where Regeneron can establish a clear clinical advantage.
- Challenge:
Payer Pressure and Drug Pricing Scrutiny
Severity:Major
Mitigation Strategy:Systematically build robust health economics and outcomes research (HEOR) data packages to clearly demonstrate the economic value and long-term cost savings of innovative medicines to payers and health systems.
Resource Limitations
Talent Gaps
- •
Specialized talent in gene therapy manufacturing, process development, and regulatory affairs.
- •
World-class computational biologists, AI/ML engineers, and data scientists to maximize the value of the Regeneron Genetics Center.
- •
Commercial and market access leaders with experience launching products in emerging markets.
High but manageable. While R&D and commercial launches are capital-intensive, Regeneron's strong profitability and balance sheet provide substantial resources for internal investment and strategic business development.
Infrastructure Needs
Expansion of biologics and gene therapy manufacturing facilities.
Investment in a scalable, high-performance computing infrastructure to support large-scale genomic data analysis and AI-driven R&D.
Growth Opportunities
Market Expansion
- Expansion Vector:
Geographic Expansion in Asia-Pacific
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Establish strategic partnerships with local players who have strong regulatory and commercial footprints. Prioritize launches of key products like Dupixent in major markets like China and Japan, adapting commercial models to local healthcare systems.
- Expansion Vector:
Label Expansion for Dupixent
Potential Impact:High
Implementation Complexity:Medium
Recommended Approach:Aggressively pursue ongoing and planned clinical trials for new indications like Chronic Obstructive Pulmonary Disease (COPD), which could significantly increase the drug's peak sales potential and solidify its blockbuster status for the next decade.
Product Opportunities
- Opportunity:
Advance Oncology Pipeline (Bispecific Antibodies)
Market Demand Evidence:High unmet need in various cancers. Oncology remains a top area of pharmaceutical R&D spending and market growth.
Strategic Fit:Excellent. Leverages core antibody engineering capabilities (VelociSuite®) and provides a major area for revenue diversification.
Development Recommendation:Prioritize late-stage assets like odronextamab and linvoseltamab for accelerated regulatory filing. Use genetic data to identify novel combination therapies that can improve efficacy.
- Opportunity:
Commercialize Gene Therapies for Rare Diseases
Market Demand Evidence:Significant unmet need for curative treatments for monogenic diseases. The gene therapy market is forecast to grow at over 19% CAGR.
Strategic Fit:High. Aligns with science-driven mission and leverages investment in the Regeneron Genetics Center for target identification.
Development Recommendation:Focus on a select portfolio of rare disease targets where the biological rationale is strongest. Consider acquiring or licensing delivery vector technology to de-risk development.
Channel Diversification
- Channel:
Digital Health & Patient Services Platforms
Fit Assessment:Excellent
Implementation Strategy:Partner with or invest in digital health startups to build an ecosystem around key therapies, offering services like remote monitoring, telehealth consultations, and personalized adherence support to improve outcomes and patient retention.
- Channel:
Real-World Evidence (RWE) Generation Partnerships
Fit Assessment:Excellent
Implementation Strategy:Form strategic partnerships with leading healthcare data providers and academic medical centers to generate and disseminate RWE that supports market access, label expansion, and physician adoption.
Strategic Partnerships
- Partnership Type:
AI Drug Discovery Collaboration
Potential Partners
- •
Exscientia
- •
Insilico Medicine
- •
Recursion
- •
Google (DeepMind)
Expected Benefits:Accelerate target identification, reduce R&D cycle times, and increase the success rate of the preclinical pipeline by combining Regeneron's genetic data with a partner's advanced AI platform.
- Partnership Type:
Co-commercialization for Pipeline Assets
Potential Partners
- •
Pfizer
- •
Merck
- •
Eli Lilly
Expected Benefits:De-risk late-stage development and maximize the global launch of new blockbuster-potential drugs outside of core therapeutic areas by leveraging the established commercial infrastructure and market access of a large pharma partner.
Growth Strategy
North Star Metric
Pipeline Velocity & Value (PVV)
As a science-led company facing patent cliffs, long-term growth is entirely dependent on the R&D engine's ability to consistently advance high-value assets through the pipeline. This metric combines the number of programs advancing each year with their risk-adjusted net present value (rNPV), focusing the entire organization on R&D productivity and innovation.
Increase the number of programs advancing from Phase 2 to Phase 3 by 15% annually over the next three years.
Growth Model
Science-Driven Innovation & Partnership-Led Commercialization
Key Drivers
- •
Proprietary technology platforms (VelociSuite®, RGC) generating novel drug targets.
- •
High R&D investment translating into a deep, homegrown pipeline.
- •
Strategic partnerships to share risk, access new capabilities, and expand commercial reach.
- •
Aggressive lifecycle management and label expansion for approved blockbusters.
Continue to internally fund early-stage R&D while actively seeking strategic partners for late-stage development and global commercialization, especially in new therapeutic areas or geographies.
Prioritized Initiatives
- Initiative:
Secure Dupixent's 'Mega-Blockbuster' Future
Expected Impact:High
Implementation Effort:Medium
Timeframe:1-3 Years
First Steps:Aggressively pursue regulatory submissions for the COPD indication globally and accelerate clinical trials for other potential label expansions identified in the pipeline.
- Initiative:
Execute EYLEA Patent Cliff Defense
Expected Impact:High (Revenue Preservation)
Implementation Effort:High
Timeframe:Immediate (0-12 Months)
First Steps:Launch a comprehensive marketing and medical education campaign for EYLEA HD, focusing on its clinical advantages. Secure broad payer contracts to minimize biosimilar switching.
- Initiative:
Accelerate Two Late-Stage Oncology Assets to Market
Expected Impact:High
Implementation Effort:High
Timeframe:2-4 Years
First Steps:Finalize Phase 3 trial data packages for linvoseltamab and odronextamab and request priority review meetings with the FDA and EMA.
- Initiative:
Operationalize AI/ML Platform for Target Discovery
Expected Impact:High (Long-term)
Implementation Effort:Medium
Timeframe:1-2 Years
First Steps:Establish a pilot project with a leading AI drug discovery firm to validate their platform using a subset of Regeneron's genetic data on a specific disease area.
Experimentation Plan
High Leverage Tests
- Test:
Value-Based Contracts for Gene Therapies
Hypothesis:Proposing outcomes-based reimbursement models to payers will accelerate market access and justify premium pricing for curative therapies.
- Test:
Decentralized Clinical Trials (DCT) for a Phase 2 study
Hypothesis:A DCT model will reduce trial timelines by 25% and increase patient diversity compared to a traditional site-based model.
- Test:
AI-based patient identification for rare disease trials
Hypothesis:Using an AI platform to scan EMR data can identify eligible patients for rare disease trials 5x faster than manual chart review.
For each experiment, define clear success metrics (e.g., time to contract, patient enrollment rate, cost per enrolled patient), establish a baseline, and measure against a control group where feasible. Utilize a 'Go/No-Go' decision framework based on predefined targets.
Continuous. R&D experiments (trial designs) follow clinical development timelines. Commercial experiments (e.g., new sales models, digital tools) should be run on a quarterly basis within specific brand teams.
Growth Team
Maintain a strong, centralized R&D organization. For commercial growth, embed cross-functional 'Pipeline Commercialization Teams' early in the development process (Phase 2). These teams should include representation from market access, medical affairs, marketing, and HEOR to shape development strategy with a commercial mindset.
Key Roles
- •
Head of Computational Biology & AI
- •
Vice President, Gene Therapy Commercialization
- •
Director, Real-World Evidence & Digital Health
- •
Head of Strategic Partnerships (Emerging Tech)
Develop a dedicated internal training program focused on data science and health economics for commercial and medical teams. Use strategic partnerships and targeted acquisitions to bring in external expertise in rapidly evolving fields like gene therapy and AI.
Regeneron's growth foundation is exceptionally strong, built upon a world-class, science-led R&D engine that has repeatedly produced blockbuster drugs. The company exhibits strong product-market fit for its core assets, EYLEA and Dupixent, and operates in a high-growth biotechnology market. Its business model, characterized by high operating leverage on patented drugs, is highly scalable. The primary challenge and strategic imperative for Regeneron is navigating the imminent patent cliff for its leading revenue-driver, EYLEA. This threat makes the next 3-5 years a critical transition period. Sustainable future growth is entirely contingent on the company's ability to execute on three core strategies: 1) Maximizing the lifecycle of its current blockbusters, particularly by securing new indications for Dupixent to turn it into a multi-decade mega-blockbuster. 2) Successfully launching its next wave of major pipeline assets, especially in oncology, to diversify revenue streams and create new growth pillars. 3) Capitalizing on its key competitive differentiator—the Regeneron Genetics Center—by fully operationalizing AI and machine learning to accelerate the discovery of next-generation therapies, including gene-based medicines. Regeneron's leadership and scientific culture are well-suited to this challenge, but success will require augmenting its capabilities in gene therapy manufacturing, computational biology, and global commercialization. The company is well-positioned for continued market leadership, provided it can successfully convert its profound scientific potential into a new portfolio of commercial products to offset forthcoming revenue erosion.
Legal Compliance
Regeneron's Privacy Notice is exceptionally comprehensive, well-structured, and demonstrates a mature understanding of the global data protection landscape. The policy is easily accessible from the website footer. Key strengths include:
- Global Scope: Explicitly addresses its various corporate entities worldwide and provides translations, acknowledging its international operations.
- Clarity and Granularity: Uses quick links to segment policies for different data subjects (Job Candidates, HCPs, Suppliers), which is a best practice for providing clear and relevant information.
- GDPR and US Law Compliance: The notice is meticulously drafted to meet the requirements of GDPR (mentioning a DPO, legal bases for processing, data subject rights) and major US state privacy laws (CCPA/CPRA, with specific disclosures on data categories sold/shared).
- Forward-Looking: Includes a dedicated 'Consumer Health Data Privacy Policy' addendum, proactively addressing new, stringent state-level health data laws in the U.S. (e.g., Washington's My Health My Data Act).
- User Rights Empowerment: Provides clear instructions and tools (a OneTrust privacy portal and a toll-free number) for users to exercise their data rights, which is a critical operational component of compliance.
A 'Terms and Conditions' document was located, accessible through the website footer. The document is clear and covers standard, essential clauses for a corporate website, including: intellectual property rights concerning website content, disclaimers of warranties, limitations of liability, and governing law (State of New York). It contains a specific and robust 'Forward-Looking Statements' disclaimer, which is critical for a publicly traded company in the biopharmaceutical industry to manage investor expectations and comply with securities regulations. The terms appropriately state that the website does not provide medical or professional services advice, a crucial disclaimer for mitigating liability.
The website employs a sophisticated cookie consent mechanism, managed through a 'Privacy Preference Center' (powered by OneTrust), accessible from the footer. A cookie banner appears upon the first visit, providing options to accept, reject, or customize settings. The key strengths are:
- Granular Control: Users can toggle consent for different categories of cookies (e.g., Performance, Functional, Targeting Cookies).
- Prior Consent: Initial analysis indicates that non-essential cookies are not loaded prior to user consent, which aligns with the strict opt-in requirements of GDPR and the ePrivacy Directive.
- Clear Information: The preference center provides descriptions of each cookie category and lists the specific cookies used.
The implementation represents a strong, compliant approach to cookie management.
Regeneron's overall data protection framework is robust and aligned with global best practices for a company of its scale and industry. The appointment of a Data Protection Officer (DPO) with a dedicated email address meets GDPR requirements. The use of the OneTrust platform for managing data subject requests operationalizes the rights granted in the privacy notice, providing a scalable and auditable system. The policy addresses international data transfers, explicitly mentioning the use of Standard Contractual Clauses (SCCs) for transfers of EEA data, which is the current legal standard. The clear separation of data processing activities (e.g., clinical trials data is governed by informed consent, not the website policy) demonstrates a nuanced and risk-aware approach to data governance.
The website demonstrates a foundational awareness of accessibility principles. The inclusion of 'Skip to Content' links is a positive indicator for users with motor disabilities who rely on keyboard navigation. Videos embedded on the site appear to be hosted on Vimeo and include controls for closed captions (CC), which is essential for users with hearing impairments. However, a comprehensive assessment of compliance with Web Content Accessibility Guidelines (WCAG) 2.1 AA standards would require a full technical audit. Without such an audit, it is impossible to verify full compliance across all criteria, such as screen reader compatibility, ARIA landmark roles, and color contrast ratios.
Industry Specific Compliance Analysis
The website's content strategy is expertly crafted to mitigate risks associated with FDA regulations on the advertising and promotion of prescription drugs. The site focuses on corporate communications, scientific leadership, investor relations, and corporate responsibility rather than direct-to-consumer (DTC) or healthcare professional (HCP) product promotion. By refraining from making specific product claims, the site avoids the requirement to provide 'fair balance,' including extensive risk information and prescribing details. Links to dedicated clinical trial and medicine-specific sites likely carry more detailed, regulated content, effectively segmenting the corporate presence from promotional activities.
The website provides a clear and direct link to an investor relations portal containing press releases, SEC filings, and event information. This structure is standard for publicly traded companies and is designed to comply with securities laws such as Regulation Fair Disclosure (Reg FD), which mandates the broad, non-exclusionary disclosure of material information. The 'Forward-Looking Statements' disclaimer in the Terms and Conditions is a critical component of this compliance framework.
The website appropriately directs users to a separate portal for information on clinical trials. This is aligned with industry best practices and legal requirements (such as the FDA Amendments Act of 2007) that mandate the registration and results reporting of clinical trials on public databases like ClinicalTrials.gov. The corporate site's role is to act as a reliable signpost, ensuring transparency without cluttering the main corporate narrative with highly specific trial data.
The Privacy Notice correctly identifies that data collected for patient support programs can include sensitive health information. Crucially, it places the legal obligation on healthcare professionals to obtain necessary patient consents before disclosing personal data to Regeneron. This is a vital legal distinction that positions Regeneron as a 'business associate' in some contexts, rather than a 'covered entity' under HIPAA, thereby clarifying its compliance obligations. The separate Consumer Health Data Privacy Policy further strengthens its posture regarding patient and consumer health information.
Compliance Gaps
- •
No publicly available Accessibility Statement detailing the company's commitment to digital accessibility and its current level of WCAG conformance.
- •
The prominence of the 'Accept Cookies' button versus other options on the cookie banner could be perceived as encouraging acceptance, a design pattern scrutinized under some EU data protection authorities' guidance.
- •
While the Privacy Notice is comprehensive, its length and complexity may pose a challenge for the average user, despite the use of quick links.
Compliance Strengths
- •
A world-class, globally-attuned Privacy Notice that is granular, specific, and kept up-to-date.
- •
Implementation of a leading privacy management platform (OneTrust) for handling cookie consent and data subject rights requests, demonstrating an operational commitment to privacy.
- •
Excellent risk mitigation strategy for pharmaceutical marketing regulations by separating corporate communications from product-specific promotion.
- •
Proactive adoption of policies for emerging US state consumer health data laws.
- •
Clear and robust legal disclaimers and forward-looking statements tailored to the biopharmaceutical and financial sectors.
Risk Assessment
- Risk Area:
Digital Accessibility
Severity:Medium
Recommendation:Commission a formal WCAG 2.1 Level AA accessibility audit from a reputable third party. Based on the audit's findings, develop a remediation plan and publish an Accessibility Statement to demonstrate commitment and mitigate legal risk from accessibility-related lawsuits under the ADA.
- Risk Area:
Cookie Banner Design
Severity:Low
Recommendation:Review the cookie banner's UI/UX to ensure that rejecting cookies is as easy and prominent as accepting them. Consider adding a 'Reject All' button with equal visual weight to the 'Accept All' button to align with the strictest interpretations of GDPR consent.
- Risk Area:
Policy Readability
Severity:Low
Recommendation:Develop a simplified, top-level summary or FAQ to accompany the full Privacy Notice. This 'layered' approach can improve user comprehension and transparency without sacrificing legal completeness.
High Priority Recommendations
Conduct a formal WCAG 2.1 AA accessibility audit to identify and remediate any compliance gaps, thereby reducing the risk of litigation and improving site usability for all individuals.
Publish a formal Accessibility Statement on the website, outlining the company's commitment to digital inclusion and providing a channel for users to report accessibility issues.
Overall, Regeneron demonstrates a highly sophisticated and mature legal compliance posture, befitting its status as a leading global biopharmaceutical company. The company's strategic legal positioning is a clear business asset, building trust with patients, HCPs, and investors, and enabling market access in heavily regulated jurisdictions. The data privacy framework is exemplary, showcasing a proactive and comprehensive approach that goes beyond mere compliance to operationalize user rights effectively. The website's content is strategically managed to mitigate significant risks associated with FDA marketing regulations. While the existing framework is exceptionally strong, the primary area for strategic improvement lies in formalizing and communicating its commitment to digital accessibility (ADA/WCAG compliance). Addressing this would further solidify its position as a responsible industry leader and reduce a tangible, albeit medium-level, legal risk.
Visual
Design System
Corporate Professional
Excellent
Advanced
User Experience
Navigation
Horizontal Mega Menu
Intuitive
Excellent
Information Architecture
Logical
Clear
Light
Conversion Elements
- Element:
Main Hero CTA ('See what drives us')
Prominence:Medium
Effectiveness:Somewhat effective
Improvement:Change copy to be more benefit-oriented and specific, such as 'Explore Our Science' or 'Discover Our Innovations'. The current text is vague.
- Element:
Section CTA ('Learn about our research and development efforts')
Prominence:Medium
Effectiveness:Effective
Improvement:The CTA is clear and well-placed. To further optimize, consider A/B testing alternative phrasing that highlights a specific achievement or area of research.
- Element:
Section CTA ('Learn about our approach to corporate responsibility')
Prominence:Medium
Effectiveness:Effective
Improvement:This CTA clearly directs users interested in the company's ethical and social initiatives. No immediate improvement is needed.
- Element:
Recruitment CTAs ('Join our team', 'Explore our clinical trials')
Prominence:High
Effectiveness:Effective
Improvement:These CTAs are well-contrasted and use clear, action-oriented language, effectively funneling potential talent and trial participants.
- Element:
News & Updates CTA ('Visit our newsroom')
Prominence:Medium
Effectiveness:Effective
Improvement:Clearly directs users to press releases and media content, which is crucial for investors and media professionals. The placement is logical.
Assessment
Strengths
- Aspect:
Strong Brand Identity & Trust
Impact:High
Description:The website exudes a professional, scientific, and trustworthy aesthetic through its consistent use of a clean layout, a defined color palette (predominantly blues, whites, and greys), and high-quality imagery. This polished presentation reinforces Regeneron's position as a leader in the biopharmaceutical industry.
- Aspect:
Clear Information Architecture
Impact:High
Description:The content is logically structured under clear top-level navigation items like 'About,' 'Science,' 'Medicines,' 'Responsibility,' and 'Stories.' This makes it easy for diverse audience segments (investors, healthcare professionals, patients, job seekers) to find relevant information quickly, reducing friction and improving user satisfaction.
- Aspect:
Compelling Visual Storytelling
Impact:Medium
Description:The use of high-quality video and authentic photography of scientists, patients, and healthcare professionals effectively communicates the human impact of Regeneron's work. The 'LIFE-CHANGING MEDICINES' and 'doing well by doing good' sections successfully convey the company's mission and values.
- Aspect:
Excellent Mobile Responsiveness
Impact:High
Description:The design adapts seamlessly to mobile devices. The navigation collapses into a clear icon, typography remains legible, and layouts reflow logically, ensuring a positive user experience across all platforms. This is critical as a significant portion of web traffic originates from mobile.
Weaknesses
- Aspect:
Generic Hero Section CTA
Impact:Low
Description:The primary call-to-action in the hero section, 'See what drives us,' is ambiguous. It doesn't set a clear expectation for the user, potentially reducing click-through rates compared to more descriptive and compelling language.
- Aspect:
Overly Dense Text in Legal/Notice Pages
Impact:Low
Description:The 'Privacy Notice' screenshot reveals large, unbroken blocks of text that are difficult to scan and comprehend. While legally necessary, the user experience could be significantly improved by better formatting, such as using accordions, shorter paragraphs, and highlighted key terms.
- Aspect:
Lack of Interactive Data Visualization
Impact:Medium
Description:For a data-driven company, there is a missed opportunity to present key statistics (e.g., pipeline progress, clinical trial data, company growth metrics) in an interactive and engaging way. Static numbers in the 'About Regeneron at a glance' section are less impactful than dynamic charts or infographics.
Priority Recommendations
- Recommendation:
A/B Test Hero Section CTAs
Effort Level:Low
Impact Potential:Medium
Rationale:Optimize the most prominent call-to-action on the homepage to increase user engagement and guide them more effectively into the site's key content funnels. Testing specific, action-oriented copy like 'Explore Our Pipeline' or 'Our Scientific Breakthroughs' against the current 'See what drives us' can yield significant improvements in click-through rates.
- Recommendation:
Enhance Readability of Text-Heavy Pages
Effort Level:Medium
Impact Potential:Low
Rationale:Improve the user experience on essential but dense pages like the Privacy Notice. Implementing UX writing principles and formatting techniques (accordions, bolding, bullet points) will improve scannability and comprehension, demonstrating a user-centric approach even on legal pages.
- Recommendation:
Introduce Interactive Content Modules
Effort Level:High
Impact Potential:High
Rationale:To better communicate the company's scientific leadership and data-driven approach, develop interactive modules for key data points. Visualizing the R&D pipeline, clinical trial results, or company growth through interactive charts and timelines will create a more engaging and memorable experience for investors and scientific professionals.
Mobile Responsiveness
Excellent
The layout adapts smoothly across different screen sizes. Content reflows logically, typography scales appropriately, and interactive elements remain easily accessible.
Mobile Specific Issues
No itemsDesktop Specific Issues
No itemsThe Regeneron website presents a world-class digital presence that successfully balances a highly professional, corporate image with compelling, human-centric storytelling. As a leading biotechnology firm, its primary audiences include investors, healthcare professionals, potential employees, and patients. The design effectively caters to these diverse groups through a sophisticated and mature design system.
1. Design System & Brand Identity:
The site's design system is advanced and consistently applied. The visual style is clean, modern, and professional, utilizing a distinct color palette of blues and whites to evoke trust, innovation, and scientific rigor. Typography is a key strength, with clear hierarchies and excellent legibility that enhances the user experience. The brand's identity as a science-led, patient-focused innovator is powerfully expressed through high-quality visuals and a consistent tone of voice.
2. Visual Hierarchy & Information Architecture:
The visual hierarchy is exceptionally effective. Key information, such as the company's mission to create 'LIFE-CHANGING MEDICINES,' is given prominence through large, impactful typography and central placement. The information architecture is logical and intuitive. The main navigation clearly segments content for different user journeys, allowing visitors to easily access information about the company's science, medicines, and corporate responsibility. This structured approach minimizes cognitive load and facilitates efficient information discovery.
3. Navigation & User Flow:
On desktop, the website employs a horizontal mega menu, which is an excellent choice for organizing the breadth of its content without overwhelming the user. Navigation paths are clear and predictable, guiding users smoothly from broad topics to more specific information. For instance, a user interested in the company's research can easily navigate from 'Science' to specific therapeutic areas. The user flow feels natural and unobstructed.
4. Mobile Responsiveness:
The mobile experience is a standout strength. The design is fully responsive, with navigation collapsing into an intuitive hamburger menu and content blocks stacking cleanly. Touch targets are appropriately sized, and performance feels snappy. This demonstrates a strong understanding of modern web standards and user behavior, ensuring accessibility for all users regardless of their device.
5. Visual Conversion & CTAs:
Calls-to-action (CTAs) are generally well-designed, using a contrasting blue color to stand out. They are strategically placed to guide users to key areas like the newsroom, career pages, and corporate responsibility sections. However, the primary hero CTA ('See what drives us') is somewhat vague and represents a missed opportunity for more direct, benefit-driven language. Enhancing this single element could significantly improve engagement from the homepage.
6. Visual Storytelling:
Regeneron excels at visual storytelling. The use of high-impact video in the hero section and throughout the site immediately engages the user. Photography focuses on real people—scientists in labs, caring healthcare professionals, and hopeful patients—which effectively humanizes the brand and its complex scientific work. This narrative approach transforms the site from a simple corporate brochure into a compelling story of scientific pursuit and human benefit.
Discoverability
Market Visibility Assessment
Regeneron has successfully positioned itself as a premier, science-first biotechnology innovator, consistently reinforcing its brand message of 'Science to Medicine'. This is evident through its homepage content, which highlights radical innovation, expert insights, and significant investment in STEM talent. The company is recognized as a leader in translating complex genetic research into FDA-approved therapies. A key pillar of its authority is the Regeneron Genetics Center (RGC), which is building one of the world's most comprehensive genomic databases and uses this data to identify new drug targets, significantly enhancing its reputation for cutting-edge R&D.
Regeneron's market visibility is strong, primarily driven by blockbuster products like EYLEA (for eye diseases) and Dupixent (for allergic/inflammatory diseases). However, it faces intense competition from global pharmaceutical giants such as Roche/Genentech, Novartis, Amgen, and Bristol-Myers Squibb. In the lucrative ophthalmology market, EYLEA is in a direct and fierce battle for market share with Roche's Vabysmo. While EYLEA has historically dominated, competitors are gaining ground. Regeneron's digital presence for these products is critical for maintaining its position against well-funded marketing from larger rivals.
For a biotechnology firm, 'customer acquisition' targets multiple distinct audiences. The digital presence shows strong potential for attracting top-tier scientific and medical talent through its emphasis on innovation and being a top employer. For Healthcare Professionals (HCPs), the site provides access to press releases on clinical trial results and scientific publications, crucial for influencing prescribing decisions. For patients, the website offers high-level information and a portal for clinical trial exploration. The potential lies in creating more targeted, educational content journeys for both HCPs and patients to guide them from disease awareness to specific treatment information.
Regeneron demonstrates a strong global presence, with offices in 12 countries and clinical trials in over 50. The website's privacy policy, available in six languages besides English, reflects this international footprint. However, the primary content appears largely US-centric. A significant strategic opportunity exists to create localized content that addresses regional healthcare protocols, regulatory news, and market-specific needs to deepen penetration and engagement in key markets outside the U.S., such as Europe and Japan where it has an established presence.
The company's digital content effectively covers its core therapeutic areas: eye diseases, allergic and inflammatory diseases, cancer, cardiovascular diseases, and genetics. The site excels in showcasing its foundational expertise in genetics through the RGC, presenting it as a core differentiator. Content series like 'Eye on the Experts' and stories on specific research programs (e.g., auditory gene therapy) demonstrate deep subject matter expertise. The coverage is robust at the scientific and corporate levels, but there is an opportunity to translate this expertise into more accessible patient-facing content.
Strategic Content Positioning
Regeneron's content strategy primarily serves audiences in the mid-to-late stages of the journey: R&D professionals, potential employees, and investors, who are well-served by scientific publications, corporate reports, and career information. The journey for HCPs is supported by clinical trial data and press releases. However, the top-of-funnel journey for patients seeking to understand their conditions is less developed. Aligning content to address early-stage patient questions and concerns could build trust and create a pathway towards brand familiarity and clinical trial participation.
Regeneron is already executing on thought leadership through its focus on the RGC, 'Eye on the Experts,' and its founder's principles. The major opportunity is to amplify this content externally. By converting internal expertise and scientific publications into more digestible formats like webinars, executive summaries for HCPs, and data visualizations, Regeneron could dominate search results for forward-looking topics like 'future of genetic medicine,' 'AI in drug discovery,' and specific rare disease research, further cementing its brand as a visionary leader.
Competitors like Novartis and Roche often have more extensive, patient-centric educational portals with interactive tools and condition-specific resources. Regeneron's content is heavily weighted towards its own science and corporate story. A significant gap is the lack of comprehensive, unbranded disease awareness content. Creating dedicated resource hubs for conditions like atopic dermatitis or wet age-related macular degeneration could capture significant patient and caregiver search traffic early in their journey, an area where competitors are often more visible.
The brand message of being a science-driven company that translates research into medicine is exceptionally consistent across the entire digital presence. From the homepage's 'SCIENCE TO MEDICINE' tagline to the detailed narratives about the RGC and in-house development pipeline, every touchpoint reinforces this core identity. This consistency is a major strength, clearly differentiating Regeneron from competitors who may have a more diversified or sales-focused message.
Digital Market Strategy
Market Expansion Opportunities
- •
Develop patient-centric, unbranded educational hubs for key therapeutic areas (e.g., ophthalmology, dermatology) to capture early-stage search interest.
- •
Create localized content microsites for key international markets (e.g., EU, Japan) featuring region-specific news, clinical trial updates, and expert perspectives.
- •
Launch a dedicated content series around emerging genetic medicines and rare diseases to establish authority and attract partnerships in these high-growth fields.
Customer Acquisition Optimization
- •
Create a secure portal for HCPs with exclusive access to detailed clinical data, webcasts with lead researchers, and prescribing information to improve engagement and trust.
- •
Enhance the visibility of patient support programs through targeted SEO and content marketing to address patient concerns about access and affordability.
- •
Optimize the 'Explore Clinical Trials' section with more intuitive search functions and patient-friendly trial summaries to increase enrollment inquiries.
Brand Authority Initiatives
- •
Amplify the profiles of key scientists (like George Yancopoulos and the RGC team) through interviews, bylined articles in major publications, and participation in high-profile industry podcasts.
- •
Systematically promote peer-reviewed publications through digital PR and targeted outreach to medical and scientific journalists to earn high-authority backlinks.
- •
Develop a comprehensive annual report on the future of biotechnology, leveraging RGC data and internal expertise to create a cornerstone thought leadership asset.
Competitive Positioning Improvements
- •
Differentiate from competitors by heavily promoting the 'homegrown' nature of nearly all its product candidates, emphasizing a consistent, integrated R&D process.
- •
Create content comparing the mechanisms of action of its therapies against competitors, targeted specifically at the sophisticated HCP audience.
- •
Leverage the RGC's leadership in genomics to position Regeneron as the most data-driven and precise drug developer in the industry.
Business Impact Assessment
Market share visibility can be tracked via Share of Voice (SoV) for key branded (EYLEA, Dupixent) and non-branded (e.g., 'wet AMD treatment') keywords against primary competitors like Roche and Novartis. Changes in search rank and traffic to product-specific pages serve as leading indicators of market penetration.
For HCPs, success can be measured by engagement with gated content, webinar registrations, and downloads of scientific papers. For patients, key metrics include traffic to disease awareness sections and conversion rates on clinical trial inquiry forms. For talent, metrics include applications originating from the career site and engagement with 'Working at Regeneron' content.
Authority is measured by the volume and quality of backlinks from reputable medical, academic, and news domains. An increase in branded search volume (people searching for 'Regeneron' directly) and media mentions related to innovation and the RGC are strong indicators of growing brand equity and trust.
Benchmark digital performance against a defined set of competitors (e.g., Amgen, Roche, Novartis) on key metrics. This includes tracking keyword rankings for competitive therapeutic areas, social media share of voice on industry topics, and the volume of positive media sentiment compared to rivals.
Strategic Recommendations
High Impact Initiatives
- Initiative:
Develop Patient-Focused Disease Education Hubs
Business Impact:High
Market Opportunity:Capture significant top-of-funnel search traffic from patients and caregivers at the beginning of their healthcare journey. Competitors are currently more active in this space, representing a key opportunity to build brand trust and preference early.
Success Metrics
- •
Organic traffic growth to new educational sections
- •
Keyword rankings for non-branded disease terms
- •
Time on page and engagement rate
- •
Click-through rate to branded product/trial pages
- Initiative:
Launch an 'HCP Knowledge Center' Portal
Business Impact:High
Market Opportunity:Strengthen relationships with healthcare professionals by providing exclusive, high-value scientific and clinical data in a secure, centralized location. This addresses the HCP need for deep, credible information beyond marketing materials and can directly influence prescribing habits.
Success Metrics
- •
Number of HCP registrations
- •
Content downloads (clinical data, whitepapers)
- •
Webinar attendance and engagement
- •
Repeat visits to the portal
- Initiative:
Amplify Regeneron Genetics Center (RGC) Thought Leadership
Business Impact:Medium
Market Opportunity:Solidify Regeneron's unique market position as the leader in genetics-driven drug discovery. By translating the RGC's complex work into accessible insights, Regeneron can attract elite talent, high-value partnerships, and positive investor attention.
Success Metrics
- •
Media mentions citing RGC research
- •
Backlinks from .edu and .gov domains
- •
Inbound partnership inquiries
- •
Social media engagement on RGC-related content
Double down on the 'Science-First, Genetics-Powered' narrative. Position Regeneron as the most innovative and scientifically rigorous company in biotechnology, using the Regeneron Genetics Center as the primary proof point. While competitors focus on broad therapeutic areas, Regeneron's strategic advantage is its proven, repeatable process of turning genetic insights into homegrown medicines. Every digital communication should reinforce this unique ability to innovate 'over and over again'.
Competitive Advantage Opportunities
- •
Leverage the RGC's vast and diverse genomic database as a key differentiator in all corporate and scientific communications, highlighting its role in accelerating drug discovery.
- •
Emphasize the 'physician-scientist' leadership to build credibility with the HCP community, showcasing a shared commitment to patient outcomes rooted in scientific understanding.
- •
Promote the high percentage of internally discovered and developed drug candidates as evidence of a superior, more integrated R&D engine compared to competitors who rely more heavily on acquisitions.
Regeneron has established a powerful digital presence that effectively communicates its core identity as a science-driven, innovative biotechnology leader. Its brand is built on a foundation of scientific rigor, exemplified by the prominent positioning of the Regeneron Genetics Center (RGC) and a consistent 'Science to Medicine' message. This strategy resonates strongly with key audiences such as investors, partners, and prospective top-tier employees.
The primary strategic challenge lies in the competitive digital landscape for its key products, particularly EYLEA, which faces intense pressure from well-funded marketing campaigns by giants like Roche. While Regeneron excels at communicating its science to a sophisticated audience, its digital presence has a significant opportunity to better engage patients and caregivers earlier in their information-seeking journey. Currently, the website is geared more towards those who are already aware of Regeneron, rather than capturing those new to a diagnosis.
To secure and expand its market leadership, Regeneron should pursue a dual-pronged digital strategy:
-
Deepen HCP Engagement: Create a dedicated, high-value digital ecosystem for Healthcare Professionals. This goes beyond press releases to offer exclusive access to data, expert discussions, and educational resources that empower clinical decision-making and build unshakeable loyalty.
-
Broaden Patient Outreach: Invest in building comprehensive, non-branded disease education hubs. By becoming a trusted source of information for patients at the point of diagnosis, Regeneron can build brand preference long before treatment decisions are made, creating a critical competitive moat and a direct channel for communicating the value of its therapies and clinical trials.
Strategic Priorities
Strategic Priorities
- Title:
Execute 'Project Diversify' to Establish a Third Revenue Pillar in Oncology
Business Rationale:The business faces a critical revenue concentration risk with over-reliance on Eylea and Dupixent. With Eylea's patent cliff imminent, establishing a new blockbuster-potential revenue stream is the highest strategic priority to ensure sustainable long-term growth and de-risk the business model.
Strategic Impact:This initiative transforms Regeneron from a company dependent on two core assets into a multi-pillar biopharmaceutical leader. It validates the R&D engine's ability to innovate beyond its initial successes and provides the necessary cash flow to fund the next generation of genetic medicines.
Success Metrics
- •
Achieve regulatory approval for two late-stage oncology assets within 36 months
- •
Generate >$1B in annual revenue from the oncology portfolio within 3 years of launch
- •
Reduce revenue concentration from Eylea & Dupixent to <60% of total revenue within 5 years
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Revenue Model
- Title:
Launch 'Franchise Fortress' Defense for the Ophthalmology Portfolio
Business Rationale:The Eylea franchise is under immediate and intense threat from both biosimilar entry and strong competition (e.g., Roche's Vabysmo). Failure to protect this core revenue stream would severely impair the company's ability to fund its R&D pipeline and future growth initiatives.
Strategic Impact:A successful defense preserves billions in annual revenue, maintains market leadership in a key therapeutic area, and provides a financial bridge to the next wave of innovative products. It demonstrates the company's ability to compete on both innovation and commercial execution.
Success Metrics
- •
Achieve a >70% conversion rate of existing Eylea patients to Eylea HD within 18 months
- •
Maintain >45% market share in the branded anti-VEGF ophthalmology market
- •
Stabilize year-over-year revenue erosion in the ophthalmology franchise to less than 15% post-biosimilar entry
Priority Level:HIGH
Timeline:Quick Win (0-3 months)
Category:Market Position
- Title:
Operationalize the Regeneron Genetics Center (RGC) with an AI-Powered Discovery Platform
Business Rationale:The RGC is Regeneron's most sustainable competitive advantage. Currently, its potential is not fully realized. Integrating a world-class AI/ML platform will exponentially increase the speed and precision of novel drug target identification, creating a near-insurmountable lead in genetics-driven medicine.
Strategic Impact:This transforms the R&D function from a highly effective but traditional process into a predictive, data-driven engine. It shortens drug discovery timelines, increases pipeline success rates, and opens new revenue opportunities in personalized medicine and strategic data partnerships.
Success Metrics
- •
Decrease average time from target identification to preclinical candidate by 30%
- •
Double the number of validated, novel drug targets entering the pipeline annually
- •
Establish one major strategic partnership with a leading AI/tech firm within 18 months
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Operations
- Title:
Establish Patient-Centric Digital Ecosystems to Build Direct Brand Preference
Business Rationale:Competitors are more effectively engaging patients and caregivers early in their journey. By creating unbranded disease education hubs and digital support tools, Regeneron can build trust and brand preference long before a treatment decision is made, creating a competitive moat that is difficult for rivals to penetrate.
Strategic Impact:This strategy shifts from a purely physician-focused model to a dual B2B/B2C approach. It captures significant market-shaping influence, improves patient adherence and outcomes, and generates valuable real-world data to inform future R&D and commercial strategies.
Success Metrics
- •
Become a top 3 organic search result for key non-branded disease terms in core therapeutic areas
- •
Increase patient enrollment in support programs by 25% year-over-year
- •
Increase inbound inquiries for clinical trial participation originating from owned digital properties by 50%
Priority Level:MEDIUM
Timeline:Strategic Initiative (3-12 months)
Category:Customer Strategy
- Title:
Launch an Exclusive 'HCP Knowledge Center' to Drive Clinical Loyalty
Business Rationale:Healthcare Professionals (HCPs) are the ultimate arbiters of product success. In a crowded market, providing them with exclusive, high-value clinical data, real-world evidence, and access to key opinion leaders in a centralized digital portal will build deep, defensible loyalty beyond the reach of traditional sales forces.
Strategic Impact:This initiative transforms the relationship with HCPs from transactional to partnership-based. It accelerates the adoption of new medicines and new indications, defends market share against competitors, and establishes Regeneron as the definitive scientific authority in its core therapeutic areas.
Success Metrics
- •
Achieve 30% registration rate among target specialist HCPs within 12 months
- •
Measure a 15% higher prescribing rate for key products among active portal users versus non-users
- •
Attain a Net Promoter Score (NPS) of +50 from portal users
Priority Level:MEDIUM
Timeline:Strategic Initiative (3-12 months)
Category:Customer Strategy
Regeneron must execute a pivotal transition from a two-blockbuster success story to a diversified biopharmaceutical leader. This requires flawlessly defending its core ophthalmology franchise through superior lifecycle management while aggressively accelerating its oncology pipeline to establish a third revenue pillar. Long-term dominance will be secured by weaponizing its unique genetic data asset with AI to create an unrivaled, predictive drug discovery engine.
The core competitive advantage to build and amplify is the vertically integrated, 'homegrown' R&D engine, supercharged by the fusion of the Regeneron Genetics Center's (RGC) massive proprietary dataset with an advanced AI platform. This creates a repeatable, predictable system for innovation that is nearly impossible for competitors to replicate.
The primary growth catalyst is the successful commercialization of the late-stage oncology pipeline. Launching new, wholly-owned blockbuster products in oncology will provide the most immediate and significant path to revenue diversification, de-risk the company from its current concentration, and fund the next generation of genetic medicine breakthroughs.