eScore
vrtx.comThe eScore is a comprehensive evaluation of a business's online presence and effectiveness. It analyzes multiple factors including digital presence, brand communication, conversion optimization, and competitive advantage.
Vertex maintains a world-class corporate digital presence with a high degree of authority, especially in its core Cystic Fibrosis (CF) market. The website's content is expertly structured for its key audiences—investors, healthcare professionals (HCPs), and patients—aligning well with their distinct search intents and journeys. Its multi-channel presence is strong, reinforced by significant media coverage of its innovations and leadership, although its direct social media engagement could be more dynamic. Content authority is exceptionally high, rooted in its scientific leadership and validated by its robust pipeline and regulatory approvals.
Exceptional content authority and domain credibility, built on a foundation of market-leading scientific innovation in cystic fibrosis and pioneering work in gene therapy.
Develop more accessible scientific content (e.g., infographics, short videos) that explains the complex mechanisms of new therapies like CASGEVY and JOURNAVX to a broader patient and caregiver audience, enhancing search intent alignment for non-experts.
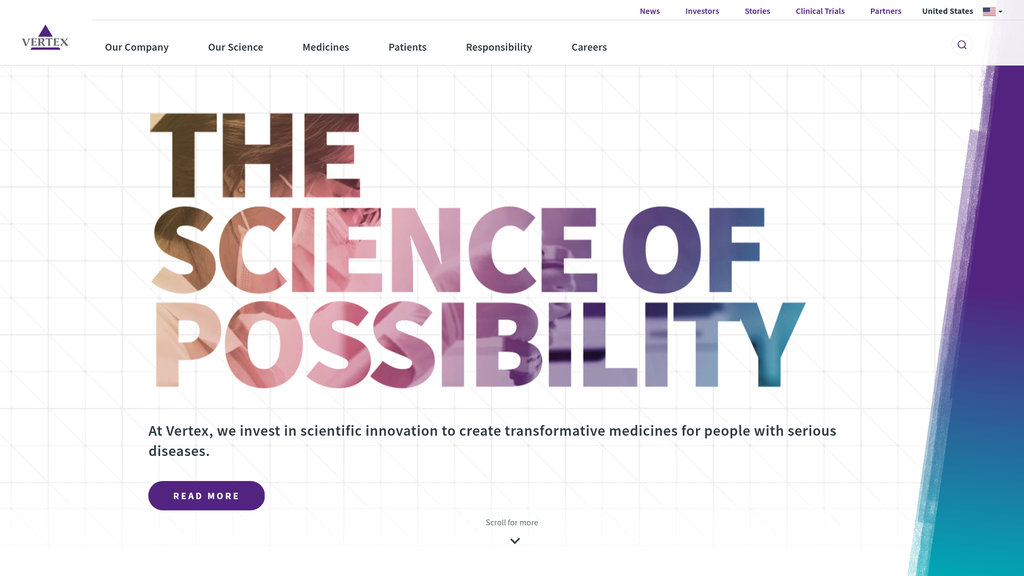
Vertex's brand messaging is remarkably consistent and powerful, centered on the compelling tagline "The Science of Possibility." The communication effectively segments audiences, delivering detailed financial and scientific data for investors and HCPs, while providing patient-centric information and clinical trial access for patients and caregivers. The brand voice successfully balances scientific authority with a compassionate, patient-focused tone. However, the reliance on generic calls-to-action like "Learn More" is a missed opportunity for more persuasive, benefit-driven language.
A highly consistent and well-defined brand message—"The Science of Possibility"—that effectively bridges its legacy in CF with its ambitious, multi-disease future.
A/B test call-to-action (CTA) copy to be more specific and benefit-oriented (e.g., 'Explore Our Pipeline's Potential' instead of 'Learn More'), which could improve engagement and better guide user journeys.
The website offers a seamless and intuitive user experience with a low cognitive load, clear navigation, and excellent mobile responsiveness. The information architecture is logical, effectively funneling diverse audiences to relevant content sections. The primary conversion points for a biopharma company—accessing clinical trial information, investor data, and scientific news—are well-executed. The primary weakness lies in the lack of interactive data visualization and the use of understated 'ghost button' styles for some secondary CTAs, which may reduce engagement.
An intuitive and clean information architecture with excellent mobile responsiveness, ensuring a low-friction experience for all user personas across devices.
Incorporate interactive content, such as a dynamic pipeline chart or visual timelines of company milestones, to make complex scientific and financial data more engaging and digestible for investor and scientific audiences.
Vertex excels in establishing credibility through a powerful hierarchy of trust signals. Third-party validation is immense, including landmark FDA approvals for first-in-class drugs like CASGEVY, prestigious scientific awards for its researchers, and consistent recognition as a top employer. The website transparently provides extensive data for investors, detailed scientific publications, and clear information on its leadership. Customer success is powerfully demonstrated through patient stories and, most importantly, the transformative clinical data of its medicines.
Overwhelming third-party validation through landmark FDA approvals, major scientific awards, and consistent positive media coverage of its innovations, creating an unimpeachable foundation of credibility.
While legal policies are robust, the cookie consent banner's 'implied consent' model is a minor gap in GDPR compliance that could be updated to an explicit opt-in/opt-out mechanism to fully align with global best practices.
Vertex's competitive moat is exceptionally strong and sustainable, anchored by its near-monopolistic dominance in the CF market which provides massive cash flow for R&D. The company has built a second major advantage by achieving the first-ever approval for a CRISPR-based therapy (CASGEVY), giving it invaluable first-mover experience in a revolutionary new modality. While revenue is currently concentrated in CF, its deep and diversified pipeline across pain, diabetes, and kidney disease demonstrates a clear strategy to build new moats. High R&D costs and complex regulatory hurdles create formidable barriers to entry for competitors.
A highly defensible and profitable monopoly in the Cystic Fibrosis market that funds a 'serial innovation' engine, enabling the creation of new, hard-to-replicate advantages like being the first-to-market with a CRISPR-based therapy.
Aggressively execute the commercial launches of CASGEVY and JOURNAVX to accelerate revenue diversification, mitigating the primary long-term strategic risk of over-reliance on the CF franchise.
Vertex's scalability is proven, having grown into a global biopharma leader on the strength of its CF franchise. The company's strong financial position and massive cash flow enable significant investment in market expansion and pipeline development. Expansion potential is vast, with a diversified late-stage pipeline targeting large markets like pain, type 1 diabetes, and kidney disease. The primary constraint on scalability is the immense complexity and cost of manufacturing and delivering cell and gene therapies like CASGEVY, which requires building a global network of specialized treatment centers.
A robust, multi-billion dollar cash flow from its core CF business that provides the capital to fund aggressive expansion into new, large therapeutic areas and invest in complex but high-potential technologies like cell and gene therapy.
Invest heavily in optimizing and scaling the manufacturing and logistics for cell and gene therapies to reduce 'vein-to-vein' time and cost, which is the primary bottleneck to the commercial scalability of CASGEVY and future curative therapies.
Vertex has a remarkably coherent and effective business model: dominate a niche, high-unmet-need disease area (CF) to generate substantial profits, then reinvest those profits to fund high-risk, high-reward R&D in new therapeutic areas to create the next wave of transformative medicines. This 'serial innovation' model is strategically focused and has been executed with exceptional discipline. Resource allocation is heavily skewed towards R&D, which is appropriate for its strategy. The company is perfectly timed with market trends toward curative and genetic medicines.
A clear, powerful, and self-funding 'serial innovation' model that uses profits from its dominant CF franchise to systematically de-risk and advance a diversified pipeline of next-generation transformative therapies.
Proactively develop innovative, value-based pricing models for high-cost curative therapies like CASGEVY to address payer pushback and ensure market access, making the revenue model more resilient to pricing pressures.
In its core CF market, Vertex's market power is nearly absolute, with dominant market share and significant pricing power. The company is now leveraging this power to enter new, more competitive markets. With CASGEVY, it has established a lead over its direct competitor (bluebird bio's Lyfgenia) in early patient uptake. The approval of JOURNAVX as a first-in-class non-opioid pain treatment allows Vertex to influence and shape a new standard of care, demonstrating significant market influence beyond its legacy franchise.
Near-absolute market dominance and pricing power in the lucrative cystic fibrosis market, which provides the strategic leverage and financial resources to enter and disrupt new therapeutic categories from a position of strength.
Develop and execute a robust health economics and outcomes research (HEOR) strategy to clearly demonstrate the long-term value and cost-effectiveness of JOURNAVX compared to cheaper but riskier opioids, thereby solidifying its pricing power in a competitive market.
Business Overview
Business Classification
Biotechnology / Pharmaceuticals
Specialty & Orphan Drugs
Healthcare
Sub Verticals
- •
Small Molecule Therapeutics
- •
Gene Therapy
- •
Cell Therapy
- •
Orphan Diseases
Mature
Maturity Indicators
- •
Fortune 500 company with multiple blockbuster drugs (e.g., TRIKAFTA® sales exceeding $8.9 billion in 2023).
- •
Consistent double-digit annual revenue growth.
- •
Robust and diversified late-stage R&D pipeline across multiple therapeutic areas.
- •
Established global commercial infrastructure and sales force.
- •
Strong balance sheet with significant cash reserves (e.g., $11.2 billion as of Dec 2024).
Enterprise
Steady & Diversifying
Revenue Model
Primary Revenue Streams
- Stream Name:
Product Sales (Cystic Fibrosis Franchise)
Description:Direct sales of its portfolio of transformative medicines that treat the underlying cause of cystic fibrosis (CF), including TRIKAFTA®/KAFTRIO®, SYMDEKO®/SYMKEVI®, ORKAMBI®, and KALYDECO®. This is the company's core revenue engine, representing the vast majority of its income.
Estimated Importance:Primary
Customer Segment:Patients with Cystic Fibrosis (via Healthcare Providers & Payers)
Estimated Margin:High
- Stream Name:
Product Sales (New & Emerging Therapies)
Description:Sales from newly launched, high-value therapies such as CASGEVY®, a gene-edited therapy for sickle cell disease and beta thalassemia, and JOURNAVX™, a non-opioid treatment for acute pain. These represent the initial phase of revenue diversification.
Estimated Importance:Secondary
Customer Segment:Patients with Sickle Cell Disease, Beta Thalassemia, and Acute Pain
Estimated Margin:High
- Stream Name:
Collaborations & Royalties
Description:Upfront payments, milestone payments, and potential future royalties from strategic collaborations with other biotechnology and pharmaceutical companies (e.g., CRISPR Therapeutics, Moderna) to co-develop and commercialize new therapies.
Estimated Importance:Tertiary
Customer Segment:Biopharmaceutical Partners
Estimated Margin:High
Recurring Revenue Components
Chronic care medication for Cystic Fibrosis (lifelong treatment)
Potential for long-term follow-up revenue from gene and cell therapies
Pricing Strategy
Value-Based & Monopoly Pricing
Premium
Opaque
Pricing Psychology
- •
Monopoly Leverage: Capitalizing on the lack of alternative treatments that address the underlying cause of CF to command high prices.
- •
High R&D Justification: Pricing reflects the massive investment and scientific innovation required to develop first-in-class, transformative therapies.
- •
Value Justification: Prices are defended based on the significant clinical benefits, improved quality of life, and long-term healthcare savings compared to managing severe disease progression.
Monetization Assessment
Strengths
- •
Dominant market position in CF (~97% market share) provides significant pricing power and predictable, high-margin revenue.
- •
Strong patent protection for key revenue drivers, delaying generic competition.
- •
Successful reimbursement negotiations with payers in major markets, ensuring broad patient access for high-cost therapies.
Weaknesses
- •
High revenue concentration on the CF franchise creates significant business risk.
- •
Premium pricing strategy attracts intense scrutiny from payers, governments, and patient advocacy groups, leading to pricing pressure and reputational risk.
- •
Complex reimbursement landscape for ultra-high-cost gene therapies like CASGEVY® could slow initial uptake.
Opportunities
- •
Successfully commercialize the non-CF pipeline (pain, diabetes, kidney disease) to diversify revenue streams.
- •
Expand geographic access to CF therapies in markets with unmet need.
- •
Leverage real-world evidence to reinforce value propositions and defend pricing with payers.
Threats
- •
Future patent expirations for blockbuster drugs like TRIKAFTA®.
- •
Increased government intervention on drug pricing in key markets like the U.S.
- •
Clinical trial failures or regulatory setbacks in the diversification pipeline.
- •
Emergence of competitive therapies, particularly in the gene therapy space for CF and other targeted diseases.
Market Positioning
Scientific Leadership & Disease-Area Dominance
Dominant (in Cystic Fibrosis)
Target Segments
- Segment Name:
Patients with Cystic Fibrosis (CF)
Description:Individuals diagnosed with the genetic disorder cystic fibrosis, with specific mutations treatable by Vertex's CFTR modulators. The company targets expansion into younger patient populations and the remaining ~10% who cannot benefit from current medicines.
Demographic Factors
All ages, from infants to adults
Predominantly affects Caucasian populations
Psychographic Factors
Seeking transformative treatments over symptomatic relief
Highly engaged with patient advocacy groups
Behavioral Factors
Adherence to lifelong, daily medication regimens
Reliance on specialty pharmacies and care centers
Pain Points
- •
Progressive decline in lung function
- •
Chronic infections and digestive issues
- •
Shortened life expectancy
- •
High treatment burden
Fit Assessment:Excellent
Segment Potential:Medium
- Segment Name:
Patients with Sickle Cell Disease (SCD) & Beta Thalassemia (TDT)
Description:A new, high-unmet-need segment for Vertex's CRISPR-based gene therapy, CASGEVY®. These are patients with severe genetic blood disorders requiring frequent transfusions and managing debilitating symptoms.
Demographic Factors
Primarily affects individuals of African, Mediterranean, and South Asian descent
Psychographic Factors
Desire for a one-time, potentially curative treatment
Willingness to undergo a complex treatment process (cell collection, chemotherapy)
Behavioral Factors
Frequent hospitalizations
Management of chronic pain crises (SCD)
Pain Points
- •
Severe, chronic pain
- •
Organ damage
- •
High burden of blood transfusions
- •
Reduced quality of life and life expectancy
Fit Assessment:Good
Segment Potential:High
- Segment Name:
Healthcare Providers (Specialists)
Description:Pulmonologists, hematologists, endocrinologists, and other specialists at major medical centers and authorized treatment centers who diagnose and treat patients with these serious diseases.
Demographic Factors
Highly trained medical professionals
Psychographic Factors
Motivated by clinical efficacy and patient outcomes
Value robust scientific data and peer-reviewed publications
Behavioral Factors
Prescribe high-value specialty drugs
Navigate complex reimbursement and prior authorization processes
Pain Points
- •
Lack of effective, disease-modifying treatments for their patients
- •
Administrative burden of prescribing and managing specialty therapies
- •
Keeping abreast of rapidly advancing science
Fit Assessment:Excellent
Segment Potential:High
Market Differentiation
- Factor:
First-in-Class & Best-in-Class Science
Strength:Strong
Sustainability:Sustainable
- Factor:
Disease-Modifying Mechanism of Action
Strength:Strong
Sustainability:Sustainable
- Factor:
Dominant Patent Estate in Core Market (CF)
Strength:Strong
Sustainability:Temporary
- Factor:
Cutting-Edge Multi-Modality R&D Pipeline
Strength:Strong
Sustainability:Sustainable
Value Proposition
We invest in scientific innovation to create transformative medicines that treat the underlying cause of serious diseases, not just the symptoms.
Excellent
Key Benefits
- Benefit:
Transforms the course of disease and improves patient quality of life
Importance:Critical
Differentiation:Unique
Proof Elements
- •
Extensive Phase 3 clinical trial data
- •
Regulatory approvals from FDA, EMA, and others
- •
Real-world evidence and patient testimonials
- Benefit:
Offers a potential one-time, curative therapy for genetic disorders (e.g., CASGEVY®)
Importance:Critical
Differentiation:Unique
Proof Elements
First-ever approval for a CRISPR-based gene-editing therapy
Pivotal trial results showing transformative outcomes
- Benefit:
Provides novel, non-opioid options for pain management
Importance:Important
Differentiation:Somewhat unique
Proof Elements
Positive Phase 2/3 data for suzetrigine (VX-548).
FDA approval for JOURNAVX™
Unique Selling Points
- Usp:
The only company with a portfolio of medicines that treat the underlying cause of cystic fibrosis for up to 90% of patients.
Sustainability:Medium-term
Defensibility:Strong
- Usp:
Pioneered the first approved CRISPR-based gene therapy in partnership with CRISPR Therapeutics.
Sustainability:Long-term
Defensibility:Moderate
- Usp:
A deep, multi-modality pipeline targeting causal human biology in areas of high unmet need.
Sustainability:Long-term
Defensibility:Strong
Customer Problems Solved
- Problem:
Progressive, life-shortening genetic diseases with limited or no treatment options that address the root cause.
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Debilitating chronic conditions (e.g., severe pain, dependence on blood transfusions) that severely impact daily life.
Severity:Critical
Solution Effectiveness:Partial
- Problem:
Over-reliance on addictive opioid medications for managing acute pain.
Severity:Major
Solution Effectiveness:Partial
Value Alignment Assessment
High
Vertex targets diseases with significant unmet needs and clear biological drivers, ensuring that successful therapies will have a substantial market and high value.
High
The company's focus on transformative, life-altering outcomes directly addresses the most critical pain points of patients and the physicians who treat them.
Strategic Assessment
Business Model Canvas
Key Partners
- •
CRISPR Therapeutics (gene editing).
- •
Moderna (mRNA therapies).
- •
Arbor Biotechnologies (gene editing).
- •
Academic research institutions and hospitals.
- •
Patient advocacy groups (e.g., Cystic Fibrosis Foundation).
Key Activities
- •
Research & Development (investing heavily, ~$3.6B in 2024).
- •
Clinical trials management (Phase 1 through 4).
- •
Global regulatory affairs and submissions
- •
Specialty pharmaceutical manufacturing
- •
Targeted sales and marketing to specialists.
Key Resources
- •
Intellectual property (robust patent portfolio).
- •
World-class scientific and clinical talent
- •
Proprietary drug discovery platforms and technologies
- •
Strong balance sheet and access to capital.
- •
Regulatory expertise
Cost Structure
- •
High R&D expenditures.
- •
Acquired In-Process R&D (AIPR&D) from acquisitions (e.g., $4.4B for Alpine).
- •
Sales, General & Administrative (SG&A) expenses to support global commercial launches.
- •
Cost of Goods Sold (COGS) for complex biologics and small molecules
Swot Analysis
Strengths
- •
Virtual monopoly in the CF market, generating substantial and predictable cash flow.
- •
Proven R&D engine with a track record of bringing transformative, first-in-class drugs to market.
- •
Deep pipeline across multiple therapeutic modalities and disease areas, promising future growth.
- •
Strong financial position with high profitability and significant cash reserves for strategic investments.
Weaknesses
- •
Over-reliance on the cystic fibrosis franchise for revenue, creating concentration risk.
- •
High drug prices attract intense public and political scrutiny, posing a risk to long-term pricing power.
- •
High-risk, high-reward R&D model where pipeline failures can significantly impact valuation.
Opportunities
- •
Successfully commercialize the non-CF pipeline (pain, T1D, kidney disease) to become a multi-franchise biopharma leader.
- •
Leverage the CASGEVY® launch to build a leadership position in the burgeoning field of gene therapy.
- •
Utilize strong cash flow for strategic M&A to acquire new technologies or late-stage assets (e.g., Alpine Immune Sciences).
- •
Expand into larger markets beyond rare diseases.
Threats
- •
Governmental drug pricing reforms in key markets (e.g., Inflation Reduction Act in the U.S.).
- •
Patent expirations on core CF products, leading to eventual generic competition.
- •
Increased competition from other biotech firms in gene therapy, pain, and other pipeline areas.
- •
Setbacks in late-stage clinical trials for key pipeline assets.
Recommendations
Priority Improvements
- Area:
Revenue Diversification Execution
Recommendation:Intensify focus on the successful commercial execution for CASGEVY® and JOURNAVX®. This includes streamlining patient access, securing broad payer coverage, and building out specialized sales and support infrastructure to accelerate uptake and de-risk the portfolio from CF dependency.
Expected Impact:High
- Area:
Value Proposition & Pricing Defense
Recommendation:Proactively develop and deploy a comprehensive value-based defense strategy. This involves investing heavily in health economics and outcomes research (HEOR) to clearly quantify the long-term cost savings and quality-of-life benefits of high-cost therapies, mitigating pushback from payers and policymakers.
Expected Impact:Medium
- Area:
Pipeline Prioritization
Recommendation:Conduct a rigorous portfolio review to prioritize late-stage assets with the highest probability of technical and regulatory success and the largest market potential. Reallocate R&D resources to accelerate the most promising programs (e.g., inaxaplin for kidney disease) to reach the market faster.
Expected Impact:High
Business Model Innovation
- •
Explore innovative pricing and reimbursement models for one-time curative therapies like CASGEVY®, such as outcomes-based annuities or milestone payments, to ease the upfront budget impact on healthcare systems.
- •
Develop a 'Vertex Digital Health' platform to provide wrap-around services for patients on chronic or complex therapies, improving adherence, collecting real-world data, and strengthening the company's value proposition beyond the pill.
- •
Establish a corporate venture arm to make strategic, early-stage investments in disruptive technologies (e.g., next-generation gene editing, AI-driven drug discovery) to fuel the future R&D pipeline.
Revenue Diversification
- •
Aggressively advance the clinical development of inaxaplin for APOL1-mediated kidney disease, a potential blockbuster opportunity in a large specialty market.
- •
Continue strategic, bolt-on acquisitions of companies with promising mid-to-late-stage assets in new therapeutic areas to supplement the internal pipeline, similar to the Alpine Immune Sciences deal for povetacicept.
- •
Evaluate opportunities to in-license assets in adjacent specialty therapeutic areas where Vertex can leverage its existing R&D and commercialization expertise.
Vertex Pharmaceuticals exemplifies a highly successful, science-driven biotechnology business model. The company has masterfully executed a strategy of achieving near-monopolistic dominance in a high-unmet-need orphan disease (Cystic Fibrosis), creating a powerful and profitable revenue engine. This core franchise provides the substantial cash flow necessary to fund a high-risk, high-reward R&D pipeline aimed at transformative therapies in new, larger markets. The current strategic inflection point for Vertex is its evolution from a single-franchise company to a diversified, multi-modality biopharmaceutical leader. The successful launches of CASGEVY® in gene therapy and JOURNAVX® in pain are the first critical tests of this transformation. The primary challenge and opportunity lie in executing this diversification strategy flawlessly. This requires not only scientific and clinical success but also navigating increasingly complex commercial and reimbursement landscapes for ultra-high-cost therapies. Future success will be defined by the company's ability to replicate its CF success in new disease areas, thereby mitigating its primary strategic vulnerability: revenue concentration.
Competitors
Competitive Landscape
Growth
Oligopoly
Barriers To Entry
- Barrier:
High R&D and Clinical Trial Costs
Impact:High
- Barrier:
Stringent Regulatory Approval Processes (FDA, EMA)
Impact:High
- Barrier:
Intellectual Property & Patent Protection
Impact:High
- Barrier:
Complex Manufacturing and Supply Chain
Impact:High
- Barrier:
Economies of Scale in Commercialization
Impact:Medium
Industry Trends
- Trend:
Rise of Cell and Gene Therapies
Impact On Business:Positive, as it aligns with Vertex's strategy (CASGEVY) and pipeline, but also increases competition from specialized biotechs.
Timeline:Immediate
- Trend:
Focus on Precision Medicine and Rare Diseases
Impact On Business:Highly positive, as this is Vertex's core business model, validating their focus on genetically defined diseases.
Timeline:Immediate
- Trend:
AI and Machine Learning in Drug Discovery
Impact On Business:Potential to accelerate R&D timelines and identify new targets, requiring investment in new technologies to keep pace.
Timeline:Near-term
- Trend:
Increased Scrutiny on Drug Pricing and Reimbursement
Impact On Business:High impact, as Vertex's high-value, transformative therapies (especially gene therapies) face significant pricing pressure and market access challenges.
Timeline:Immediate
- Trend:
Shift towards In-Vivo Gene Editing
Impact On Business:Represents the next frontier. Vertex's expertise in ex-vivo editing (CASGEVY) provides a foundation, but they will need to invest or partner to compete in the in-vivo space.
Timeline:Long-term
Direct Competitors
- →
bluebird bio
Market Share Estimate:Significant competitor in the gene therapy space for hemoglobinopathies.
Target Audience Overlap:High
Competitive Positioning:Pioneer in lentiviral vector-based gene therapies for severe genetic diseases, with a focus on sickle cell disease and beta-thalassemia.
Strengths
- •
First-mover experience with gene therapy commercialization (ZYNTEGLO for beta-thalassemia, LYFGENIA for sickle cell).
- •
Established network of qualified treatment centers.
- •
Deep expertise in lentiviral vector technology.
Weaknesses
- •
Financial instability and smaller scale compared to Vertex.
- •
LYFGENIA carries a black box warning for hematologic malignancy, a potential disadvantage against CASGEVY.
- •
Slower initial patient uptake for LYFGENIA compared to CASGEVY.
Differentiators
Uses a lentiviral vector gene addition approach, contrasting with Vertex/CRISPR's gene-editing mechanism.
- →
CRISPR Therapeutics
Market Share Estimate:Direct partner and potential future competitor in the CRISPR gene-editing space.
Target Audience Overlap:High
Competitive Positioning:A leading gene-editing company focused on developing transformative gene-based medicines using its proprietary CRISPR/Cas9 platform.
Strengths
- •
Co-developer of CASGEVY, sharing in its success and validating their platform.
- •
Strong intellectual property foundation in CRISPR/Cas9 technology.
- •
Expanding pipeline in immuno-oncology, autoimmune diseases, and cardiovascular indications.
Weaknesses
- •
Heavily reliant on the success of the CASGEVY launch for revenue.
- •
Still in a high cash-burn phase, with significant R&D expenses.
- •
Less commercial infrastructure and experience compared to Vertex.
Differentiators
Pure-play focus on advancing the CRISPR/Cas9 platform across multiple therapeutic areas, both ex-vivo and in-vivo.
- →
Sionna Therapeutics
Market Share Estimate:Emerging clinical-stage competitor in Cystic Fibrosis.
Target Audience Overlap:High
Competitive Positioning:A clinical-stage company focused on developing highly effective and differentiated treatments for cystic fibrosis by targeting the NBD1 domain of the CFTR protein.
Strengths
Novel mechanism of action that could potentially offer improved efficacy over existing CFTR modulators.
Acquired a portfolio of CF drug candidates from AbbVie, suggesting a serious attempt to challenge Vertex's dominance.
Weaknesses
- •
Very early stage compared to Vertex's established CF franchise.
- •
High risk of clinical trial failure; many previous attempts by others (including AbbVie) to compete with Vertex in CF have failed.
- •
Significantly fewer financial and commercial resources.
Differentiators
Focus on the NBD1 domain of the CFTR protein, a different approach than Vertex's modulators.
- →
Novo Nordisk
Market Share Estimate:Major competitor in the future Type 1 Diabetes (T1D) market.
Target Audience Overlap:Medium
Competitive Positioning:A global leader in diabetes care (especially insulin) that is aggressively investing in stem cell-based therapies to develop a curative treatment for Type 1 Diabetes.
Strengths
- •
Dominant global market leader in diabetes with extensive commercial infrastructure and physician relationships.
- •
Significant investment in cell therapy R&D, with the stated goal of finding a cure for T1D.
- •
Strategic partnerships and acquisitions to accelerate their cell therapy programs.
Weaknesses
Cell therapy programs for T1D are still in early-to-mid clinical stages.
Primary expertise is in metabolic diseases, with less of a track record in cell and gene therapy compared to specialized biotechs.
Differentiators
Leveraging a century of diabetes expertise to inform their cell therapy development.
Exploring encapsulation devices to protect transplanted cells from the immune system.
Indirect Competitors
- →
AbbVie Inc.
Description:While AbbVie has discontinued its direct CFTR modulator program, it remains a major player in managing CF symptoms through products like CREON (pancreatic enzyme replacement therapy) and its robust community support programs (e.g., AbbVie CF Scholarship).
Threat Level:Low
Potential For Direct Competition:Low, given their recent clinical trial failures and program discontinuation in CFTR modulators.
- →
Alnylam Pharmaceuticals
Description:A leader in RNA interference (RNAi) therapeutics for rare genetic diseases. While not in Vertex's core markets, their focus on genetic medicine and innovative platform technology makes them a competitor for talent, capital, and potentially future therapeutic areas.
Threat Level:Low
Potential For Direct Competition:Medium, as their RNAi platform could be applied to diseases Vertex may target in the future.
- →
Sarepta Therapeutics
Description:A leader in precision genetic medicine for rare neuromuscular diseases (e.g., Duchenne muscular dystrophy). They compete in the broader genetic medicine space for investment and regulatory attention.
Threat Level:Low
Potential For Direct Competition:Low, as their focus is primarily on neuromuscular diseases.
Competitive Advantage Analysis
Sustainable Advantages
- Advantage:
Market Dominance in Cystic Fibrosis (CF)
Sustainability Assessment:Highly sustainable in the near-to-medium term due to a portfolio of approved, life-changing drugs and a deep pipeline for the remaining patient population.
Competitor Replication Difficulty:Hard
- Advantage:
First-Mover Advantage in CRISPR-based Therapeutics
Sustainability Assessment:Sustainable, as CASGEVY is the first approved CRISPR-based therapy, giving Vertex invaluable experience with manufacturing, commercialization, and regulatory processes for this new class of medicine.
Competitor Replication Difficulty:Hard
- Advantage:
Serial Innovation & R&D Engine
Sustainability Assessment:Moderately sustainable. The company has a proven track record of translating scientific insights into approved medicines, but the high-risk nature of biotech R&D means future success is not guaranteed.
Competitor Replication Difficulty:Medium
- Advantage:
Strong Financial Position
Sustainability Assessment:Highly sustainable. Strong cash flow from the CF franchise enables significant R&D investment, business development, and commercialization of new products.
Competitor Replication Difficulty:Hard
Temporary Advantages
{'advantage': 'Exclusivity for JOURNAVX (New Pain Class)', 'estimated_duration': '5-10 years, depending on patent life and the emergence of competing non-opioid pain medications with novel mechanisms.'}
Disadvantages
- Disadvantage:
Revenue Concentration on Cystic Fibrosis
Impact:Major
Addressability:Moderately
- Disadvantage:
High Price Point of Curative Therapies
Impact:Major
Addressability:Difficult
- Disadvantage:
Complexity of Cell & Gene Therapy Manufacturing
Impact:Major
Addressability:Moderately
Strategic Recommendations
Quick Wins
- Recommendation:
Aggressively market JOURNAVX's non-opioid mechanism to healthcare systems and providers to accelerate adoption and capitalize on the first-mover advantage in its class.
Expected Impact:High
Implementation Difficulty:Easy
- Recommendation:
Launch targeted educational campaigns for physicians and patients on the benefits and logistics of CASGEVY to streamline the patient journey and outpace bluebird bio's LYFGENIA.
Expected Impact:High
Implementation Difficulty:Moderate
Medium Term Strategies
- Recommendation:
Accelerate clinical development of the Type 1 Diabetes cell therapy program to establish a lead against formidable competitors like Novo Nordisk.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Invest in or partner with companies specializing in in-vivo gene editing and novel delivery technologies (e.g., next-gen LNPs) to build capabilities for the next wave of genetic medicines.
Expected Impact:High
Implementation Difficulty:Moderate
- Recommendation:
Expand the pipeline in rare renal diseases, leveraging internal expertise from the CEO and building another potential franchise outside of CF.
Expected Impact:Medium
Implementation Difficulty:Difficult
Long Term Strategies
- Recommendation:
Systematically pursue diversification through strategic M&A, targeting companies with promising mid-to-late stage assets in adjacent specialty therapeutic areas to reduce long-term reliance on the CF franchise.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Develop a global access strategy for CASGEVY in low- and middle-income countries where sickle cell disease is prevalent, potentially through innovative financing models or partnerships.
Expected Impact:Medium
Implementation Difficulty:Difficult
Position Vertex as the undisputed leader in 'curative-intent' medicines for genetically defined diseases, leveraging the success stories of CF and CASGEVY to build a master brand synonymous with transformative, one-time therapies.
Differentiate through a 'serial innovation' model: dominating a disease area (CF), using the proceeds to pioneer a new therapeutic modality (CRISPR gene editing), and repeating this cycle in new areas (T1D, pain, renal disease). This narrative highlights both scientific mastery and strategic capital allocation.
Whitespace Opportunities
- Opportunity:
Develop therapies for the remaining ~10% of CF patients who cannot benefit from current CFTR modulators.
Competitive Gap:This is an unmet need that falls directly within Vertex's core expertise, where competitors have failed to gain a foothold.
Feasibility:High
Potential Impact:High
- Opportunity:
Apply CRISPR/Cas9 expertise to other rare genetic hematological or liver disorders.
Competitive Gap:Leverages the validated platform and manufacturing know-how from CASGEVY to expand into new rare diseases with less direct competition than in areas like oncology.
Feasibility:Medium
Potential Impact:High
- Opportunity:
Develop novel, non-addictive treatments for chronic pain.
Competitive Gap:While JOURNAVX addresses acute pain, the chronic pain market is vast and still heavily reliant on opioids and older drug classes, representing a significant unmet need.
Feasibility:Medium
Potential Impact:High
Vertex Pharmaceuticals has established a formidable competitive position, transitioning from a company defined by its near-monopoly in Cystic Fibrosis (CF) to a diversified, multi-franchise leader in specialty medicines. Their core sustainable advantage is a proven R&D engine capable of 'serial innovation'—achieving market dominance in one area and leveraging that financial strength to pioneer new therapeutic classes. The recent landmark approval of CASGEVY, the first CRISPR-based therapy, co-developed with CRISPR Therapeutics, exemplifies this strategy and places them at the forefront of the gene-editing revolution.
In their new markets, the competitive landscape is intense. In sickle cell disease, their direct competitor is bluebird bio's LYFGENIA. While both are one-time gene therapies, Vertex's CASGEVY appears to have an early commercial edge and lacks the black box warning present on LYFGENIA's label. The primary challenge in this space is not just competition, but the immense cost and logistical complexity of commercializing these multi-million dollar therapies. In Type 1 Diabetes, Vertex faces future competition from established giants like Novo Nordisk, who are heavily investing in curative cell therapies and possess deep market expertise. The pain market presents an opportunity to diversify revenue with JOURNAVX, a first-in-class non-opioid, but will require significant commercial effort to displace entrenched standards of care.
The greatest strategic threat to Vertex is its long-term reliance on the CF franchise. While currently a massive advantage, this revenue concentration is a vulnerability that the company is actively addressing through its pipeline diversification. The barriers to entry in the biopharmaceutical industry—including staggering R&D costs, complex regulation, and intellectual property hurdles—protect incumbents like Vertex but do not eliminate the threat of disruptive innovation.
Strategic priorities must focus on flawless execution of the CASGEVY and JOURNAVX launches, acceleration of the T1D and renal disease pipelines, and continued investment in next-generation platforms like in-vivo gene editing. By positioning itself as the leader in transformative, curative-intent medicines for genetically-defined diseases, Vertex can build on its legacy in CF to define the next era of biotechnology.
Messaging
Message Architecture
Key Messages
- Message:
We invest in scientific innovation to create transformative medicines for people with serious diseases.
Prominence:Primary
Clarity Score:High
Location:Homepage Hero Banner
- Message:
THE SCIENCE OF POSSIBILITY
Prominence:Primary
Clarity Score:Medium
Location:Homepage Hero Banner (Headline)
- Message:
We’re focused on discovering, developing and producing innovative medicines so people with serious diseases can lead better lives.
Prominence:Secondary
Clarity Score:High
Location:Homepage 'OUR SCIENCE' section
- Message:
Our company is built on an inclusive culture that brings together the best and brightest... to improve the lives of people with serious diseases.
Prominence:Secondary
Clarity Score:High
Location:Homepage 'Working at Vertex' section
- Message:
Guided by our uncompromising commitment to patients, we are committed to going the distance...
Prominence:Tertiary
Clarity Score:High
Location:Homepage 'Featured stories' section
The messaging hierarchy is clear and effective. It correctly prioritizes the core mission of scientific innovation for transformative medicines. Supporting messages logically flow from this primary theme, branching into the specific areas of science, patient stories, corporate responsibility, and career opportunities. This creates a cohesive and easily understood narrative for the visitor.
Messaging is exceptionally consistent across the homepage and the CEO's biography. The core concepts of 'scientific innovation,' 'transformative medicines,' and a focus on 'serious diseases' are repeated, reinforcing the brand's central strategy. The values mentioned in corporate responsibility sections align with the culture described in the CEO's bio and career pages.
Brand Voice
Voice Attributes
- Attribute:
Authoritative & Scientific
Strength:Strong
Examples
- •
pioneering research and discovery of medicines
- •
Vertex’s clinical-stage pipeline is advancing best in class and/or first in class approaches
- •
advancing multiple modalities including small molecule, mRNA, biologics, cell and gene therapies
- Attribute:
Patient-Centric & Compassionate
Strength:Strong
Examples
- •
improve the lives of people with serious diseases
- •
uncompromising commitment to patients
- •
help make new medicines a reality
- Attribute:
Ambitious & Forward-Looking
Strength:Strong
Examples
- •
THE SCIENCE OF POSSIBILITY
- •
we will never stop fighting until we discover cures
- •
Our future journey in cystic fibrosis
- Attribute:
Professional & Corporate
Strength:Moderate
Examples
- •
We've announced our Q2 financial results
- •
Prioritizing corporate responsibility
- •
Through our transformative medicines for patients, we drive value for our company, shareholders and society.
Tone Analysis
Scientific and professional, establishing credibility and expertise.
Secondary Tones
- •
Hopeful
- •
Compassionate
- •
Inspirational
Tone Shifts
The tone shifts from highly scientific and corporate in news releases and investor sections to more personal and compassionate in the 'Featured Stories' and patient-focused sections.
Voice Consistency Rating
Excellent
Consistency Issues
No itemsValue Proposition Assessment
Vertex leverages unparalleled scientific innovation to discover and develop transformative, often first-in-class, medicines that treat the underlying cause of serious diseases for which there are limited or no therapeutic options.
Value Proposition Components
- Component:
Scientific Leadership & Innovation
Clarity:Clear
Uniqueness:Somewhat Unique
Details:Emphasis on 'pioneering research' and 'first in class approaches' is strong. While many biotechs claim innovation, Vertex backs this up with specific examples like CASGEVY (gene-editing) and a 'first new class of pain medicines'.
- Component:
Focus on High Unmet Need / Serious Diseases
Clarity:Clear
Uniqueness:Unique
Details:The relentless focus on 'serious diseases' like cystic fibrosis, sickle cell disease, and type 1 diabetes is a core differentiator, positioning them in specialty markets with high impact potential.
- Component:
Transformative Patient Outcomes
Clarity:Clear
Uniqueness:Somewhat Unique
Details:The goal is not just incremental improvement but creating 'transformative medicines' that allow people to 'lead better lives'. This outcome-focused language is powerful and patient-centric.
Vertex effectively differentiates itself by focusing on its strategy of targeting the underlying cause of serious genetic diseases, a more fundamental approach than merely treating symptoms. This is powerfully demonstrated by its leadership in cystic fibrosis. The 'Science of Possibility' tagline captures this ambitious, boundary-pushing ethos. Their expansion into cutting-edge modalities like gene-editing further separates them from traditional pharmaceutical companies.
The messaging positions Vertex as a dominant leader in the cystic fibrosis market and a serious, scientifically-driven pioneer in other complex therapeutic areas. By highlighting major scientific awards and landmark approvals, the messaging aims to place Vertex in an elite category of innovators, competing not just with other biotech firms but shaping the future of medicine itself.
Audience Messaging
Target Personas
- Persona:
Investors & Shareholders
Tailored Messages
- •
We've announced our Q2 financial results
- •
Through our transformative medicines for patients, we drive value for our company, shareholders and society.
- •
Vertex has more than doubled its employee base and the number of medicines it offers, significantly expanded its R&D pipeline...
Effectiveness:Effective
- Persona:
Patients & Caregivers
Tailored Messages
- •
Find a Vertex clinical trial
- •
Pain can impact anyone...
- •
Living life with cystic fibrosis: The Circle of Care grant
- •
We’re focused on discovering, developing and producing innovative medicines so people with serious diseases can lead better lives.
Effectiveness:Somewhat Effective
- Persona:
Scientific & Medical Community
Tailored Messages
- •
Vertex researcher receives the 2025 Canada Gairdner International Award...
- •
We announced updated data for our investigational cell therapy for the treatment of type 1 diabetes...
- •
Vertex’s clinical-stage pipeline is advancing best in class and/or first in class approaches...
Effectiveness:Effective
- Persona:
Potential Employees & Talent
Tailored Messages
- •
Our company is built on an inclusive culture that brings together the best and brightest...
- •
Vertex has consistently been recognized as a best place to work...
- •
Careers
- •
Students
Effectiveness:Effective
Audience Pain Points Addressed
- •
Living with a serious, life-altering disease with few treatment options.
- •
The physical and emotional burden of chronic conditions like cystic fibrosis and pain.
- •
The search for hope and scientific breakthroughs for genetic disorders.
Audience Aspirations Addressed
- •
The possibility of living a better, fuller life despite a serious diagnosis.
- •
Access to cutting-edge, transformative treatments.
- •
The desire for cures, not just symptom management.
- •
For scientific talent, the aspiration to work on groundbreaking science that makes a real-world impact.
Persuasion Elements
Emotional Appeals
- Appeal Type:
Hope
Effectiveness:High
Examples
- •
THE SCIENCE OF POSSIBILITY
- •
Our future journey in cystic fibrosis
- •
Volunteers who participate in clinical trials help make new medicines a reality.
- Appeal Type:
Inspiration
Effectiveness:Medium
Examples
Meet the 2025 Vertex Science Leaders Scholarship recipients
Alex Smith's story
Social Proof Elements
- Proof Type:
Awards & Recognition
Impact:Strong
Details:Prestigious awards like the 'Canada Gairdner International Award' and CEO recognitions from TIME and Fortune build immense credibility.
- Proof Type:
Financial Performance
Impact:Strong
Details:Announcements of quarterly financial results signal stability and success to investors and the market.
- Proof Type:
Scientific Validation
Impact:Strong
Details:Announcing updated data at major conferences like the 'American Diabetes Association Scientific Sessions' demonstrates progress and peer validation.
- Proof Type:
Media & Third-Party Endorsements
Impact:Strong
Details:The CEO's bio is rich with mentions in Fortune, TIME, Barron's, and Business Insider, lending third-party credibility to the company's leadership and vision.
Trust Indicators
- •
CEO's detailed medical and scientific credentials (M.D., FASN).
- •
Specific names of approved medicines (TRIKAFTA®, CASGEVY®).
- •
Publication of Corporate Responsibility Reports.
- •
Clear sections for distinct audiences like Investors and Media.
Scarcity Urgency Tactics
Not Applicable. This type of messaging is inappropriate and not used in the context of a biopharmaceutical company focused on serious diseases.
Calls To Action
Primary Ctas
- Text:
Read More
Location:Homepage Hero
Clarity:Clear
- Text:
Learn More
Location:Multiple sections (Our Science, Pain Game Plan, News)
Clarity:Clear
- Text:
Careers
Location:Working at Vertex section
Clarity:Clear
- Text:
Find a Vertex clinical trial
Location:Dedicated section on Homepage
Clarity:Clear
The CTAs are clear, functional, and effectively segment traffic to the appropriate site sections. However, they are generic ('Learn More,' 'Read More') and lack persuasive, benefit-oriented language. They function primarily as navigation rather than compelling invitations to engage, representing a missed opportunity to reinforce value at the point of action.
Messaging Gaps Analysis
Critical Gaps
There is a lack of clear, simplified explanations of the company's complex science for a non-expert (patient/caregiver) audience on the homepage. The link between 'The Science of Possibility' and how the science actually creates that possibility is not fully closed.
Patient stories, while present in the 'Stories' section, are not integrated into the primary homepage narrative. A direct patient voice or testimonial on the main page is missing.
Contradiction Points
No itemsUnderdeveloped Areas
The 'Pain Game Plan' featuring Alex Smith feels somewhat disconnected from the core 'deep science' narrative. While a good awareness campaign, its strategic link to Vertex's scientific mission could be better articulated on the homepage.
The value proposition for why a potential employee should choose Vertex over another top biotech firm could be more explicitly stated, moving beyond 'inclusive culture' to the unique scientific challenges and opportunities they offer.
Messaging Quality
Strengths
- •
Exceptional consistency in core messaging across all content.
- •
Strong projection of authority, leadership, and scientific credibility.
- •
Effective use of social proof (awards, financial data, regulatory approvals) to build trust and validate claims.
- •
Clear segmentation of messaging for different key audiences (Investors, Media, Patients, Talent).
Weaknesses
- •
Over-reliance on generic CTAs like 'Learn More' which are functional but uninspiring.
- •
The human/patient element is secondary to the corporate/scientific narrative on the homepage.
- •
The messaging can be dense and heavy on corporate jargon, potentially alienating audiences not familiar with the biopharma industry.
Opportunities
- •
Develop more accessible content (e.g., infographics, short videos) that explains the mechanism of action for their key therapies to a lay audience.
- •
Integrate patient testimonials directly into the value proposition on the homepage to create a more immediate emotional connection.
- •
Create more benefit-driven CTA copy (e.g., 'Explore Our Pipeline's Potential' instead of 'Learn More').
Optimization Roadmap
Priority Improvements
- Area:
Homepage Narrative
Recommendation:Integrate a patient-focused element directly into the hero section, such as a powerful quote or short video clip. This will immediately bridge the gap between the high-level 'Science of Possibility' and the real-world human impact.
Expected Impact:High
- Area:
Call-to-Action Language
Recommendation:A/B test CTA copy across the site. Replace generic phrases with more active, benefit-oriented language. For the 'Our Science' section, test 'See Our Science in Action' or 'Discover Our Breakthroughs' against 'Learn More'.
Expected Impact:Medium
- Area:
Scientific Communication
Recommendation:Create a dedicated, visually engaging sub-section or page linked from the homepage that explains Vertex's core scientific platforms (e.g., gene-editing, small molecules) in simplified terms for patients and the general public.
Expected Impact:Medium
Quick Wins
Rewrite the CTAs on the homepage to be more engaging.
Add a powerful, patient-centric subheading below the main 'THE SCIENCE OF POSSIBILITY' headline.
Long Term Recommendations
Develop comprehensive, patient-centric content hubs for each major disease area (beyond CF) that integrate scientific information, clinical trial updates, patient stories, and resources.
Launch a brand-level campaign that more deeply explores the human stories behind 'The Science of Possibility,' showing the 'before and after' of their transformative medicines.
Vertex Pharmaceuticals has crafted a powerful, consistent, and authoritative strategic messaging platform that effectively positions it as a leader in scientific innovation for serious diseases. The messaging architecture is logical, prioritizing its core mission and consistently reinforcing it across all content, from the homepage to executive leadership bios. The brand voice is a well-calibrated mix of scientific authority and patient-centric compassion, which successfully builds credibility with professional audiences (investors, medical community) while establishing an emotional foundation with patients and caregivers.
The core value proposition is clear and differentiated, focusing on tackling the underlying cause of diseases through pioneering science. This is powerfully substantiated by extensive social proof, including prestigious awards, landmark drug approvals, and consistent media recognition. However, the communication strategy shows an opportunity for optimization in its audience engagement tactics. While effectively catering to investors and scientists, the messaging could forge a stronger, more immediate connection with the patient community by elevating human stories from a secondary 'Stories' section into the primary homepage narrative. The website's CTAs are functional but generic, missing an opportunity to use more persuasive, benefit-driven language to guide user journeys.
Overall, Vertex's messaging is a high-performing strategic asset that drives its corporate objectives. The primary opportunity for evolution lies in making its complex science more accessible and its human impact more visible, thereby broadening its appeal and deepening its emotional resonance with the very people its science aims to serve.
Growth Readiness
Growth Foundation
Product Market Fit
Strong
Evidence
- •
Dominant market position in Cystic Fibrosis (CF) with a portfolio of five approved, disease-modifying therapies (TRIKAFTA®, etc.) treating up to 90% of patients.
- •
Full-year 2024 product revenue of $11.02 billion, a 12% increase from 2023, primarily driven by the CF franchise.
- •
Landmark approvals for CASGEVY™, the first CRISPR-based gene-edited therapy, for sickle cell disease (SCD) and transfusion-dependent beta-thalassemia (TDT), addressing a significant unmet need.
- •
Recent U.S. approval of JOURNAVX™ (suzetrigine) for moderate-to-severe acute pain, marking a new, non-opioid class of medicine.
- •
Positive physician and patient reception for CFTR modulators, which have become the standard of care.
Improvement Areas
- •
Demonstrate strong commercial uptake and value proposition for new launches (CASGEVY, JOURNAVX) to replicate the success seen in CF.
- •
Generate long-term real-world evidence for CASGEVY to solidify its position as a curative therapy and justify its high cost.
- •
Clearly differentiate JOURNAVX against established, cheaper pain alternatives, as it failed to show superiority over Vicodin in key secondary endpoints.
Market Dynamics
Biotechnology market expected to grow ~13% from 2024 to 2025. Specific therapeutic areas show strong growth: CF market CAGR ~12-14% , Non-opioid Pain market CAGR ~7-8% , and Gene Therapy market is rapidly expanding.
Mature in core CF market; Growing in new specialty areas like gene therapy (SCD/TDT), non-opioid pain, and cell therapy for Type 1 Diabetes.
Market Trends
- Trend:
Shift towards curative therapies (gene and cell therapy).
Business Impact:Positions Vertex's CASGEVY and Type 1 Diabetes pipeline at the forefront of innovation but also introduces manufacturing and reimbursement complexity.
- Trend:
Increased payer scrutiny on high-cost drugs.
Business Impact:Creates significant market access challenges for new, high-priced therapies like CASGEVY, requiring robust value demonstration.
- Trend:
Demand for safer, non-addictive pain management solutions.
Business Impact:Creates a major market opportunity for JOURNAVX, addressing the opioid crisis.
- Trend:
AI and advanced analytics accelerating R&D.
Business Impact:Opportunity to shorten drug discovery timelines and improve clinical trial success rates, but requires investment in new capabilities.
Excellent. Vertex is capitalizing on its CF cash flow to launch into high-growth, high-need areas (gene therapy, non-opioid pain) at a time of major scientific and market shifts.
Business Model Scalability
Medium
Extremely high fixed costs in R&D and manufacturing setup. Variable costs are relatively low per unit post-approval for small molecules, but remain high for complex cell/gene therapies.
High for approved small molecule drugs (like the CF portfolio), where revenue growth significantly outpaces incremental costs. Lower for cell/gene therapies due to complex, patient-specific manufacturing and logistics.
Scalability Constraints
- •
Complex and highly regulated manufacturing processes for cell and gene therapies (CASGEVY).
- •
Requirement to build out a global network of specialized treatment centers for CASGEVY.
- •
Navigating disparate global regulatory and reimbursement systems for each new product and indication.
Team Readiness
Strong. CEO Reshma Kewalramani has a proven track record of scientific and commercial success, leading the company's diversification strategy.
Proven structure for developing and commercializing specialty drugs, but now being tested by the need to manage multiple, distinct therapeutic areas and modalities simultaneously.
Key Capability Gaps
- •
Building deep commercial expertise in the primary care-adjacent acute pain market, which differs significantly from the rare disease model.
- •
Scaling patient services and logistics for one-time curative therapies (CASGEVY) versus chronic treatments (CF portfolio).
- •
Managing the complexities and costs of global cell and gene therapy manufacturing and delivery.
Growth Engine
Acquisition Channels
- Channel:
Medical Science Liaisons (MSLs) & Key Opinion Leader (KOL) Engagement
Effectiveness:High
Optimization Potential:Medium
Recommendation:Develop tailored KOL engagement plans for each new therapeutic area (pain, nephrology, diabetes) to build advocacy and inform clinical practice.
- Channel:
Payer & Health System Negotiations
Effectiveness:Medium
Optimization Potential:High
Recommendation:Develop innovative pricing and value-based agreements for CASGEVY and JOURNAVX to accelerate market access and secure favorable formulary placement.
- Channel:
Patient Advocacy Group Partnerships
Effectiveness:High
Optimization Potential:Medium
Recommendation:Deepen partnerships beyond CF to groups in SCD, TDT, and Type 1 Diabetes to support patient identification, education, and access to clinical trials.
- Channel:
Physician Education & Medical Congress Presence
Effectiveness:High
Optimization Potential:Medium
Recommendation:Launch targeted digital and in-person education campaigns for JOURNAVX, focusing on its novel mechanism of action and non-opioid profile to a broad audience of surgeons and pain specialists.
Customer Journey
Complex multi-stakeholder journey: Patient Diagnosis -> Physician Awareness -> Prescription Decision -> Payer Authorization -> Pharmacy/Treatment Center Fulfillment -> Patient Adherence/Infusion.
Friction Points
- •
Securing reimbursement and prior authorization from payers for high-cost therapies.
- •
Patient and physician logistical burden for complex treatments like CASGEVY (cell collection, conditioning regimen, infusion).
- •
Competition from established, generic, or lower-cost alternatives, especially in the pain market.
Journey Enhancement Priorities
{'area': 'Payer Authorization', 'recommendation': 'Deploy dedicated field reimbursement specialists and digital prior authorization support tools to streamline the approval process for physicians.'}
{'area': 'CASGEVY Treatment Logistics', 'recommendation': "Create a 'white-glove' patient concierge service to manage all aspects of the treatment journey, from travel to scheduling at qualified treatment centers."}
Retention Mechanisms
- Mechanism:
Disease-modifying efficacy (CF Portfolio)
Effectiveness:High
Improvement Opportunity:Continue lifecycle management with next-generation therapies like ALYFTREK to maintain the standard of care.
- Mechanism:
Curative Potential (CASGEVY)
Effectiveness:High
Improvement Opportunity:Publish long-term follow-up data demonstrating durable, life-changing benefits to reinforce its value proposition.
- Mechanism:
Patient Support Programs
Effectiveness:Medium
Improvement Opportunity:Expand support programs to address the unique needs of patients in new disease areas, focusing on adherence, financial assistance, and mental health.
Revenue Economics
Excellent for the CF franchise with high gross-to-net margins. For new products, CASGEVY has a very high price point but also high costs of goods sold (COGS) and profit sharing with CRISPR Therapeutics. JOURNAVX economics will depend heavily on payer negotiations and market share capture.
Not a standard metric. A proxy would be 'Return on R&D and Commercialization Investment', which has been exceptionally high for the CF franchise. The goal is to replicate this model in new therapeutic areas.
High. The company generates substantial revenue ($11B+ in 2024) from a focused commercial infrastructure targeting specialized diseases.
Optimization Recommendations
- •
Streamline manufacturing processes for CASGEVY to reduce COGS over time.
- •
Secure broad reimbursement contracts for JOURNAVX early to maximize launch trajectory.
- •
Pursue geographic expansion for all approved products to leverage existing R&D investments across more markets.
Scale Barriers
Technical Limitations
- Limitation:
Manufacturing complexity of cell and gene therapies.
Impact:High
Solution Approach:Invest in dedicated manufacturing facilities (e.g., partnership with Lonza ) and process automation to improve yield, reduce costs, and ensure supply.
- Limitation:
Scientific risk in the R&D pipeline.
Impact:High
Solution Approach:Diversify the pipeline across multiple modalities and disease areas to mitigate the impact of individual trial failures (e.g., discontinuation of VX-264 for T1D ).
Operational Bottlenecks
- Bottleneck:
Global supply chain for patient-specific therapies.
Growth Impact:Limits the rate of patient treatment for CASGEVY.
Resolution Strategy:Digitize the chain of identity and custody for cell therapies; strategically expand the network of qualified treatment centers globally.
- Bottleneck:
Navigating complex global reimbursement processes.
Growth Impact:Slows revenue uptake in new markets.
Resolution Strategy:Build out local market access teams with deep expertise in each target country's health technology assessment (HTA) and pricing systems.
Market Penetration Challenges
- Challenge:
Competition from large pharma and specialized biotechs.
Severity:Major
Mitigation Strategy:Focus on being first-in-class or best-in-class, building deep scientific moats, and using strategic M&A (e.g., Alpine Immune Sciences acquisition) to bolster the pipeline.
- Challenge:
Demonstrating value of high-cost therapies to budget-constrained health systems.
Severity:Critical
Mitigation Strategy:Generate compelling health economic outcomes research (HEOR) data showing long-term cost savings and quality of life improvements.
- Challenge:
Patent expirations for foundational CF drugs in the long term.
Severity:Major
Mitigation Strategy:Continuously innovate with next-generation products (e.g., ALYFTREK) to extend franchise durability and successfully diversify revenue streams.
Resource Limitations
Talent Gaps
- •
Experts in cell and gene therapy manufacturing and quality control.
- •
Commercial leaders with experience in launching products in broad, competitive markets (acute pain).
- •
Regulatory affairs specialists with expertise in novel modalities.
Substantial capital required for late-stage clinical trials, global product launches, and strategic acquisitions.
Infrastructure Needs
Expansion of dedicated cell and gene therapy manufacturing capacity.
Investment in digital infrastructure to manage complex patient journeys and supply chains.
Growth Opportunities
Market Expansion
- Expansion Vector:
Geographic expansion of new products (CASGEVY, JOURNAVX, ALYFTREK).
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Prioritize markets with established reimbursement pathways for innovative medicines (e.g., EU5, Japan) followed by strategic entry into markets with high patient prevalence (e.g., Middle East for SCD ).
- Expansion Vector:
Indication expansion for pipeline assets (e.g., suzetrigine for neuropathic pain).
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Aggressively pursue Phase 3 programs for suzetrigine in diabetic peripheral neuropathy, leveraging its Breakthrough Therapy designation.
Product Opportunities
- Opportunity:
Successful development of the inaxaplin program for APOL1-mediated kidney disease (AMKD).
Market Demand Evidence:AMKD is a significant cause of kidney disease with no targeted treatments.
Strategic Fit:Strong fit with Vertex's strategy of targeting genetically validated diseases with high unmet need.
Development Recommendation:Focus on completing enrollment for the pivotal Phase 3 trial to support potential accelerated approval.
- Opportunity:
Advancement of the Type 1 Diabetes (T1D) cell therapy program (zimislecel).
Market Demand Evidence:Potentially curative therapy for a major chronic disease.
Strategic Fit:Represents a transformative, high-risk/high-reward opportunity in line with Vertex's mission.
Development Recommendation:Complete pivotal trial enrollment and prepare for global regulatory submissions in 2026, while concurrently working on next-generation approaches that don't require immunosuppression.
- Opportunity:
Development of povetacicept for IgA Nephropathy following the Alpine acquisition.
Market Demand Evidence:Significant need for new therapies in immune-mediated kidney diseases.
Strategic Fit:Diversifies the pipeline into immunology and strengthens the late-stage renal portfolio.
Development Recommendation:Expedite the Phase 3 RAINIER study and prepare for potential accelerated approval.
Channel Diversification
- Channel:
Digital Health & Telemedicine Integration.
Fit Assessment:Good fit for supporting patient monitoring and adherence in chronic diseases.
Implementation Strategy:Partner with leading digital health platforms to integrate patient support resources and provide remote monitoring solutions for post-treatment follow-up.
Strategic Partnerships
- Partnership Type:
Acquisition of late-stage assets or smaller biotechs.
Potential Partners
Companies with promising assets in rare genetic diseases, immunology, or neurology.
Expected Benefits:Bolster the late-stage pipeline, de-risk reliance on internal R&D, and enter new therapeutic areas, as demonstrated by the Alpine Immune Sciences acquisition.
- Partnership Type:
Technology platform licensing.
Potential Partners
Companies with novel drug delivery systems (e.g., for in-vivo gene editing) or AI-driven drug discovery platforms.
Expected Benefits:Enhance R&D capabilities, accelerate discovery timelines, and enable next-generation therapeutic approaches.
Growth Strategy
North Star Metric
Annualized Revenue from non-Cystic Fibrosis Products
Directly measures the success of the company's critical diversification strategy, which is the primary driver of long-term, sustainable growth and risk mitigation.
Achieve >25% of total revenue from non-CF products within the next 5 years.
Growth Model
Serial Innovation & Diversification Growth Model
Key Drivers
- •
Cash flow from the dominant CF franchise.
- •
Disciplined R&D investment in genetically validated targets.
- •
Successful execution of global commercial launches for new products.
- •
Strategic M&A to acquire external innovation.
Continue to reinvest CF profits into advancing the late-stage pipeline (pain, T1D, AMKD). Build out distinct, world-class commercial and medical affairs teams for each new therapeutic franchise. Systematically evaluate and execute on external M&A and licensing opportunities.
Prioritized Initiatives
- Initiative:
Maximize the U.S. commercial launch of JOURNAVX (suzetrigine) for acute pain.
Expected Impact:High
Implementation Effort:High
Timeframe:0-12 months
First Steps:Finalize payer contracting strategy and deploy a highly trained sales force targeting key hospital systems and surgical centers.
- Initiative:
Accelerate global patient access and treatment for CASGEVY.
Expected Impact:High
Implementation Effort:High
Timeframe:0-24 months
First Steps:Focus on activating the remaining qualified treatment centers and streamlining the patient onboarding and reimbursement process in the U.S. and EU.
- Initiative:
Complete enrollment for pivotal trials in AMKD (inaxaplin) and T1D (zimislecel).
Expected Impact:High
Implementation Effort:Medium
Timeframe:0-18 months
First Steps:Expand clinical trial site capacity and launch patient awareness campaigns to accelerate recruitment for these key pipeline assets.
Experimentation Plan
High Leverage Tests
{'area': 'Commercial Model', 'experiment': 'Pilot a digital-first engagement model for JOURNAVX with a segment of physicians to test effectiveness versus traditional field sales.'}
{'area': 'Market Access', 'experiment': 'Test outcomes-based reimbursement models with a select group of payers for CASGEVY, linking payment to long-term patient outcomes.'}
For commercial tests, track prescription lift, physician adoption rates, and marketing ROI. For market access tests, measure time-to-authorization, contract uptake, and impact on budget predictability for payers.
Quarterly review of ongoing experiments and prioritization of new tests based on strategic launch priorities.
Growth Team
Franchise-based model, with dedicated 'mini-CEO' general managers for each major therapeutic area (CF, Pain, Genetic Therapies, Renal, etc.) who own the P&L and strategy for their franchise.
Key Roles
- •
General Manager, Pain Franchise
- •
Head of Cell & Gene Therapy Patient Services
- •
VP of Global Market Access & Pricing
- •
Head of Corporate Development & Strategy (focused on M&A)
Actively recruit talent from companies with successful launch experience in competitive primary care/specialty markets. Develop an internal training curriculum focused on value-based care, health economics, and the unique challenges of marketing curative therapies.
Vertex Pharmaceuticals is at a pivotal growth inflection point, successfully executing a multi-year strategy to diversify beyond its dominant Cystic Fibrosis (CF) franchise. The company's growth foundation is exceptionally strong, built on a highly profitable core business that is funding a deep and diversified late-stage pipeline in high-value, specialty markets. The recent approvals of CASGEVY (gene therapy for SCD/TDT) and JOURNAVX (non-opioid for acute pain) mark the successful beginning of this new chapter.
The primary growth engine is no longer just expanding the CF market but successfully launching these new assets and advancing the next wave of potential blockbusters in kidney disease (AMKD), Type 1 Diabetes, and immunology. The company's 'Serial Innovation' model—using profits from one breakthrough to fund the next—is working. However, this diversification brings significant new challenges. The key scale barriers are not primarily technical R&D, but the operational and commercial complexities of vastly different markets and therapeutic modalities. Scaling the bespoke, high-touch model required for a curative gene therapy like CASGEVY is fundamentally different from launching a small molecule into the competitive acute pain market.
Key growth opportunities are clear: 1) Flawless commercial execution of CASGEVY and JOURNAVX globally. 2) Achieving positive pivotal trial data for inaxaplin (AMKD) and zimislecel (T1D), which would be transformative. 3) Continuing to use its strong balance sheet for strategic M&A to acquire external innovation, as seen with the Alpine deal for povetacicept.
The recommended growth strategy centers on solidifying its position as a multi-franchise, specialty pharma leader. The North Star Metric must shift from CF-centric measures to 'Annualized Revenue from non-CF Products' to align the entire organization on the diversification imperative. Success will be defined by Vertex's ability to build and scale distinct commercial and operational capabilities for each new therapeutic area while maintaining its unparalleled culture of scientific innovation.
Legal Compliance
Vertex maintains a comprehensive and geographically-aware 'Privacy Notice' easily accessible from the website footer. The policy is well-structured, detailing the types of personal data collected, processing purposes, data sharing practices, and international data transfer mechanisms. Crucially, it includes specific sections for different legal jurisdictions, such as the EEA/UK and a dedicated 'U.S. Privacy Notice' that addresses rights under various state laws including California's CPRA. This demonstrates a sophisticated and proactive approach to multi-jurisdictional privacy compliance. The notice covers data from website visitors, healthcare professionals, clinical trial participants, and others, indicating a robust understanding of its diverse data processing activities.
The 'Terms of Use' are prominently linked in the footer and are thorough for a company in the biotechnology sector. Key strengths include a very clear and explicit disclaimer that the website's content does not constitute medical advice, which is a critical risk mitigation measure. It also contains standard, enforceable clauses regarding intellectual property (protecting trademarks for its various approved medicines), limitations of liability, and disclaimers of warranties. The terms specify that Massachusetts law governs any disputes, providing a clear legal venue. The language is professional and effectively sets user expectations and legal boundaries.
The website deploys a cookie consent banner upon the initial visit. However, the mechanism is based on an 'implied consent' model, stating 'By continuing to use this site, you agree to our use of cookies,' with only a single 'Accept' button. This approach is not fully compliant with the GDPR, which requires explicit, affirmative, and granular consent. The banner lacks a visible 'Reject' or 'Manage Preferences' button, forcing users to either accept all cookies or navigate to the detailed Cookie Policy to understand how to opt-out. While a 'Cookie Policy' link is present, the initial consent mechanism represents a significant compliance gap for EU users.
Vertex's overall data protection posture is strong, anchored by its detailed and region-specific Privacy Notice. The company demonstrates a clear understanding of its obligations under GDPR and various US state privacy laws, outlining user rights and data handling procedures. They specify that data may be shared with service providers under restrictive terms and transferred internationally with appropriate safeguards. The primary weakness lies in the cookie consent implementation, which does not meet the high standards for explicit consent required by GDPR, creating a mismatch between their robust policy documentation and their practical website implementation.
The website shows a foundational awareness of accessibility needs. The inclusion of a 'Skip to main content' link is a positive indicator for users of screen readers and keyboard-only navigation. The general site structure appears to use headings and clear layouts, which benefits accessibility. However, without a formal accessibility statement or a full WCAG audit, it is difficult to assess full compliance. Given the increasing legal requirements for healthcare-related websites to adhere to WCAG 2.1 AA standards, this is an area for proactive improvement to mitigate legal risk and ensure equitable access to information.
As a global pharmaceutical company, Vertex operates under intense scrutiny from regulatory bodies like the FDA and EMA. The website's content is strategically positioned to mitigate regulatory risk. It carefully avoids promotional language for investigational drugs and maintains a clear distinction between corporate/investor information and patient/medical information. The inclusion of a dedicated 'Forward-Looking Statements' disclaimer for investors is a best practice for a publicly-traded company. However, the industry is highly sensitive to advertising violations; a recent MHRA ruling against Vertex in the UK for a LinkedIn post highlights the extreme care required in all digital communications to avoid language that could be deemed promotional to the public. This underscores the constant risk in this area, even for a well-resourced company.
Compliance Gaps
- •
The cookie consent banner uses an 'implied consent' model and lacks an explicit 'Reject' or 'Manage Preferences' option, failing to meet GDPR's stringent requirements for consent.
- •
Absence of a public-facing Accessibility Statement detailing the website's commitment and conformance to WCAG standards.
- •
The handling of any health-related information submitted through general contact forms could pose a HIPAA risk if not managed under a strict protocol, although the primary website seems to avoid collecting PHI directly.
Compliance Strengths
- •
Comprehensive, multi-jurisdictional Privacy Notice with specific addenda for the US (CPRA) and EEA (GDPR).
- •
Clear and robust 'Terms of Use' that include a critical 'Not Medical Advice' disclaimer.
- •
Strong separation of corporate/investor communications from scientific/patient information, including a 'Forward-Looking Statements' safe harbor.
- •
Demonstrates a deep internal focus on compliance, as evidenced by public information about its Office of Business Integrity and Ethics.
Risk Assessment
- Risk Area:
Cookie Consent & GDPR
Severity:High
Recommendation:Immediately update the cookie consent banner to an explicit opt-in model. It must feature equally prominent 'Accept' and 'Reject' buttons, and a link to 'Manage Preferences' allowing users to provide granular consent for different cookie categories. Failure to comply can result in significant fines from EU data protection authorities.
- Risk Area:
Digital Advertising and Promotion
Severity:High
Recommendation:Conduct regular audits of all digital content, including social media, press releases, and investor materials, to ensure strict compliance with FDA and EMA regulations on drug promotion. All communications must be carefully tailored to the intended audience (e.g., investors vs. the general public) to avoid claims of off-label promotion or advertising prescription-only medicines to consumers, as highlighted by the recent UK MHRA finding.
- Risk Area:
Website Accessibility (ADA/WCAG)
Severity:Medium
Recommendation:Commission a formal third-party audit of the website against WCAG 2.1 AA standards. Publish an Accessibility Statement outlining the findings and the company's commitment to digital inclusion. This proactively mitigates litigation risk and enhances corporate social responsibility.
- Risk Area:
Data Subject Rights Management
Severity:Low
Recommendation:While policies are in place, ensure that the internal processes for handling data subject access, deletion, and correction requests under GDPR and CPRA are streamlined, tested, and can be executed within the legally mandated timeframes.
High Priority Recommendations
- •
Overhaul the cookie consent mechanism to provide clear 'Accept' and 'Reject' options, meeting GDPR's explicit consent standard.
- •
Reinforce internal review protocols for all external digital communications to prevent language that could be construed as promotional, particularly for prescription-only medicines.
- •
Initiate a formal website accessibility audit to ensure compliance with WCAG 2.1 AA standards and publish a corresponding Accessibility Statement.
Vertex Pharmaceuticals has established a sophisticated and mature legal compliance framework, particularly in its data privacy governance and terms of use. The company's legal documentation reflects a deep understanding of the complex, multi-jurisdictional regulatory landscape it navigates as a global biotechnology leader. This strong foundation is a strategic asset, fostering trust with partners, investors, and the medical community. However, there is a notable disconnect between its well-drafted policies and the practical implementation of its cookie consent mechanism, which presents a significant and immediate compliance risk under GDPR. Furthermore, while the website's messaging is carefully managed to align with strict pharmaceutical marketing regulations, the high-stakes environment means any misstep in digital communication can lead to regulatory action, as recently demonstrated. By addressing the identified gaps in cookie consent and web accessibility, Vertex can further solidify its legal positioning, reduce its risk profile, and enhance its reputation as a responsible and trustworthy market leader.
Visual
Design System
Corporate Modern
Excellent
Advanced
User Experience
Navigation
Horizontal Top Bar (Sticky)
Intuitive
Excellent
Information Architecture
Logical
Clear
Light
Conversion Elements
- Element:
Homepage 'Learn More' CTA (Hero Section)
Prominence:High
Effectiveness:Effective
Improvement:The 'Learn More' text could be more specific to guide user expectations, e.g., 'Explore Our Science' or 'Our Research Focus'.
- Element:
'Find a Vertex clinical trial' CTA
Prominence:High
Effectiveness:Effective
Improvement:The design is strong. Consider adding a short sub-headline to directly address a key audience, like 'Patients and Caregivers: See how you can participate'.
- Element:
Differentiated CTAs ('Careers' vs. 'Students')
Prominence:Medium
Effectiveness:Somewhat Effective
Improvement:The visual treatment (ghost button style for 'Students') lessens its prominence. Using two solid, but distinct, button styles could create a stronger visual balance and appeal to both audiences equally.
- Element:
Footer Navigation Links (News, Investors, Stories)
Prominence:Low
Effectiveness:Effective
Improvement:These are appropriately placed and serve as a secondary navigation route. No immediate improvement needed as they fulfill their purpose for users who scroll to the bottom.
Assessment
Strengths
- Aspect:
Strong Brand Identity & Visual Cohesion
Impact:High
Description:The website masterfully employs Vertex's brand colors (deep purple, vibrant teals/greens) and typography. The use of dynamic, abstract gradient shapes creates a sophisticated, scientific, and forward-looking aesthetic that is consistent across all pages. This builds immediate brand recognition and trust.
- Aspect:
Clear Visual Hierarchy & Scannability
Impact:High
Description:The site uses a combination of large, impactful headlines, ample white space, and well-structured content blocks. This makes it easy for diverse audiences (investors, patients, scientists) to scan pages and quickly find relevant information, reducing cognitive load.
- Aspect:
High-Quality Imagery and Visuals
Impact:Medium
Description:The photography and graphics are professional and human-centric, effectively telling the story of the people behind the science and the patients they serve. The hero banner's 'The Science of Possibility' text treatment is visually engaging and memorable.
- Aspect:
Intuitive Navigation
Impact:High
Description:The main navigation is clear, concise, and uses audience-centric labels ('Our Science', 'Medicines', 'Patients'). The sticky header ensures it's always accessible without being intrusive, facilitating easy exploration of the site.
Weaknesses
- Aspect:
Understated Secondary CTAs
Impact:Medium
Description:Some secondary and tertiary calls-to-action utilize a 'ghost button' or outline style (e.g., 'Students', 'View All Stories'). This can reduce their visual weight and click-through rates compared to the more prominent, solid-background CTAs.
- Aspect:
Generic CTA Microcopy
Impact:Low
Description:Several primary buttons use generic 'Learn More' text. While common, this is a missed opportunity for more descriptive, action-oriented language that could better set user expectations and improve engagement by aligning more closely with the specific content.
- Aspect:
Lack of Interactive Data Visualization
Impact:Medium
Description:For a company at the forefront of science, the presentation of data and pipeline information could be more dynamic. Incorporating interactive charts, timelines, or diagrams would enhance user engagement and make complex information more digestible for investor and scientific audiences.
Priority Recommendations
- Recommendation:
A/B Test CTA Button Styles and Microcopy
Effort Level:Low
Impact Potential:Medium
Rationale:Optimize conversion by testing solid vs. ghost button styles for key secondary CTAs. Simultaneously, test more descriptive button text (e.g., 'Explore our Pipeline' instead of 'Learn More') to increase click-through rates by providing clearer user intent.
- Recommendation:
Develop Interactive Content for Key Sections
Effort Level:High
Impact Potential:High
Rationale:Transform static content in the 'Our Science' and 'Investors' sections into interactive experiences. An interactive pipeline chart or a visual timeline of company milestones would significantly boost engagement, improve comprehension of complex data, and reinforce Vertex's position as a scientific innovator.
- Recommendation:
Enhance Visual Storytelling on Key Pages
Effort Level:Medium
Impact Potential:Medium
Rationale:Incorporate more patient-focused video testimonials or short documentary-style content. While the current imagery is strong, dynamic video content would create a more profound emotional connection, humanizing the brand's mission to 'create transformative medicines'.
Mobile Responsiveness
Excellent
The design adapts seamlessly to mobile viewports. The screenshots show a logical reflowing of content, with font sizes and spacing adjusted appropriately. The primary navigation condenses into a standard, accessible hamburger menu.
Mobile Specific Issues
No itemsDesktop Specific Issues
No itemsThe Vertex Pharmaceuticals website (vrtx.com) is a world-class example of corporate digital presence in the biopharmaceutical industry. It successfully balances a highly professional and innovative brand image with a user-centric information architecture tailored to a diverse set of stakeholders including patients, healthcare professionals, investors, and prospective employees.
1. Design System Coherence and Brand Identity Expression:
The website's design system is mature and consistently applied. The visual language is defined by a sophisticated color palette of purple and teal gradients, sharp sans-serif typography, and generous use of white space. This creates a modern, clean, and scientific aesthetic that aligns perfectly with Vertex's brand identity as a leader in innovation. The tagline, "The Science of Possibility," is visually brought to life through abstract, fluid graphics and bold typographic treatments, reinforcing the company's forward-thinking mission.
2. Visual Hierarchy Effectiveness and Information Architecture:
The visual hierarchy is exceptionally clear. A logical flow is established on each page, guiding the user from high-level, impactful statements in the hero sections to more detailed information blocks. Key headlines are given significant visual weight, making the pages easily scannable. The information architecture is intuitive, with top-level navigation items like 'Our Science,' 'Medicines,' and 'Patients' catering directly to the primary user personas. This logical content organization ensures a low cognitive load, allowing users to find information efficiently.
3. Navigation Patterns and User Flow Optimization:
The site employs a sticky horizontal navigation bar on desktop, which is a standard and effective pattern for corporate sites. It remains visible as the user scrolls, providing constant access to main site sections. The user flows are clear and logical. For instance, the homepage effectively funnels different user types toward relevant content, such as directing potential job applicants to 'Careers' or researchers to 'Our Science', while giving prominence to the critical path of finding information on clinical trials.
4. Mobile Responsiveness and Cross-Device Experience:
Based on the consistent layout structure, the site is built with a mobile-first approach. The design gracefully collapses for smaller screens, with content stacking vertically in a single, easy-to-scroll column. Touch targets are appropriately sized, and the navigation transitions to a clean hamburger menu, ensuring a seamless and fully functional experience across all devices. This is crucial for audiences like healthcare professionals who may access information on the go.
5. Visual Conversion Elements and Call-to-Action Effectiveness:
Primary calls-to-action (CTAs) are visually prominent, using solid color fills that contrast well with their backgrounds. The 'Find a Vertex clinical trial' CTA is particularly well-executed, placed within a visually distinct container that draws the user's eye. However, there is a weakness in the inconsistent styling of secondary CTAs. The use of 'ghost buttons' (outline style) for actions like 'Students' and 'View All Stories' diminishes their visual prominence and could lead to lower engagement compared to their solid counterparts. Furthermore, the microcopy on several buttons is a generic 'Learn More', which is a missed opportunity for more compelling, action-oriented language.
6. Visual Storytelling and Content Presentation:
The website effectively uses visual storytelling to humanize its complex scientific work. The homepage hero immediately establishes the brand's ambitious mission. Further down, sections like 'Featured stories' and the use of authentic, high-quality photography of both researchers and real people help build an emotional connection. The biography page for the CEO is clean and professional, presenting a large volume of text in a readable, single-column format, which aids focus. The overall narrative positions Vertex not just as a drug manufacturer, but as a company of innovators dedicated to solving serious diseases.
Discoverability
Market Visibility Assessment
Vertex maintains a dominant brand authority, primarily built on its near-monopolistic position in the Cystic Fibrosis (CF) market. This is reinforced by a strong narrative around scientific innovation and patient-centricity. The high visibility of its CEO, Reshma Kewalramani, and numerous corporate awards solidify its thought leadership. However, this authority is nascent in new, highly competitive therapeutic areas like acute pain, sickle cell disease, and type 1 diabetes, where it must be actively established against entrenched players.
In the digital landscape for CF, Vertex's market share visibility is exceptionally high, capturing the vast majority of branded and high-intent non-branded search traffic. For its newer therapies—CASGEVY for sickle cell disease and JOURNAVX for acute pain—its visibility is growing but challenged. It competes with established biotechnology firms like Bluebird Bio in gene therapy and large pharmaceutical companies in the crowded pain market. Digital share-of-voice for these new areas is a critical battleground.
For a biotechnology firm, 'customer acquisition' translates to engaging Healthcare Professionals (HCPs), patients, and caregivers. The website effectively supports this through dedicated sections for clinical trials, scientific news, and patient stories. The 'Pain Game Plan' campaign featuring Alex Smith is a prime example of a direct-to-patient strategy aimed at building awareness and driving conversations with HCPs for JOURNAVX, a crucial step for market penetration.
As a global entity, Vertex's digital presence must support market access and educational efforts worldwide. The primary .com website serves as the global hub, but strategic success hinges on tailoring content and messaging to align with regional regulatory approvals and healthcare systems. The digital strategy must evolve to support launches in Europe and other key markets, moving beyond a US-centric model.
The website demonstrates excellent coverage of its core and pipeline topics. It provides deep, authoritative content on Cystic Fibrosis. Crucially, it is building out content ecosystems for its new focus areas: gene editing for sickle cell disease, non-opioid pain management, and cell therapy for type 1 diabetes. This comprehensive coverage is essential for establishing expertise and capturing search interest across the full spectrum of its innovative science.
Strategic Content Positioning
Content is well-structured to serve multiple audiences at different stages. For patients and caregivers, 'Stories' and disease information build awareness. Clinical trial details cater to those in the consideration phase. For HCPs and investors, the clear pathways to news releases, scientific publications, and financial data support evaluation and decision-making. The content strategy effectively maps to these distinct, high-stakes customer journeys.
Vertex is an established thought leader in CF. The primary opportunity is to replicate this model in its new therapeutic areas. This involves digitally amplifying scientific presentations, profiling key researchers (beyond the CEO), and creating in-depth educational content on the underlying science of CRISPR (for CASGEVY) and NaV1.8 inhibition (for JOURNAVX). This will build a moat of scientific credibility that competitors will find difficult to replicate.
In the gene therapy space for sickle cell, competitors like Bluebird Bio are also strong on patient-centric narratives. Vertex can differentiate by creating more accessible scientific content that explains the 'how' behind CRISPR/Cas9 technology. For acute pain, there is a massive opportunity to own the narrative around 'post-surgical non-opioid recovery,' filling a significant content gap and addressing societal concerns about opioid addiction.
The core brand message, 'The Science of Possibility,' is powerful and consistently woven throughout the digital presence. It effectively bridges the company's legacy in CF with its ambitious pipeline. The messaging triad of relentless scientific innovation, patient impact, and corporate responsibility is clear and consistently reinforced across all major sections of the website, from the homepage to leadership bios.
Digital Market Strategy
Market Expansion Opportunities
- •
Develop dedicated, in-depth content hubs for each new disease area (Pain, T1D, SCD/Thalassemia) to serve as authoritative digital resources for patients, caregivers, and HCPs.
- •
Launch targeted digital awareness campaigns for newly approved drugs (JOURNAVX, CASGEVY) in key international markets, aligned with regulatory approvals and reimbursement decisions.
- •
Forge digital partnerships with patient advocacy groups in new disease areas to build trust and extend reach to highly engaged communities.
Customer Acquisition Optimization
- •
Create a secure, gated portal for HCPs providing exclusive access to detailed clinical data, trial results, and medical education resources for new therapies.
- •
Utilize targeted digital advertising on professional networks (e.g., LinkedIn, Doximity) to reach specialists and prescribers with educational content.
- •
Optimize clinical trial recruitment pages for search engines to attract qualified patient volunteers directly, potentially reducing trial timelines and costs.
Brand Authority Initiatives
- •
Launch a thought leadership content series (e.g., videos, articles) profiling Vertex scientists and their research to humanize the brand and showcase internal expertise.
- •
Amplify Vertex's presence and presentations at major medical and scientific conferences through real-time digital updates, interviews, and content summaries.
- •
Develop and publish a comprehensive annual 'Innovation Report' that details pipeline progress and scientific strategy, reinforcing its position as a leader.
Competitive Positioning Improvements
- •
For CASGEVY, create a distinct digital narrative focused on the revolutionary potential of CRISPR as a 'one-time' curative therapy, differentiating it from other gene therapy approaches.
- •
For JOURNAVX, position it as the leading edge of a paradigm shift in pain management, emphasizing the 'non-addictive, non-opioid' mechanism to appeal to both clinicians and patients concerned with the opioid crisis.
- •
Actively monitor the digital share-of-voice against key competitors (e.g., Bluebird Bio, major pain medication manufacturers) in each new market and adjust content strategy to capture emerging keywords and topics.
Business Impact Assessment
Market share will be indicated by the digital share-of-voice for non-branded, disease-specific keywords (e.g., 'sickle cell gene therapy,' 'non-opioid acute pain relief') against competitors. Growth in branded search volume for new products like CASGEVY and JOURNAVX will be a primary leading indicator of market adoption and successful brand building.
Key metrics include HCP engagement rates with online educational materials, lead quality from clinical trial interest forms, and referral traffic from patient advocacy and professional medical sites. Growth in organic traffic to new disease-state hubs will signal successful audience capture.
Authority will be measured by the volume and quality of media mentions related to Vertex's research, citations of its scientific content in academic and medical forums, and organic search rankings for high-level scientific and disease-related topics. The ranking and visibility of key opinion leaders and company scientists are also crucial.
Establish benchmarks for keyword rankings, share-of-voice, and media sentiment against primary competitors in each therapeutic area. For CASGEVY, this is Bluebird Bio's Lyfgenia. For JOURNAVX, this includes both generic opioids and emerging non-opioid alternatives. Tracking these benchmarks will provide a clear view of market penetration and competitive encroachment.
Strategic Recommendations
High Impact Initiatives
- Initiative:
Launch Disease-Specific Digital Centers of Excellence
Business Impact:High
Market Opportunity:Establishes Vertex as the primary educational authority in new, competitive markets, attracting high-intent audiences (patients & HCPs) early in their journey.
Success Metrics
- •
Organic traffic growth to new content hubs
- •
Top 3 search rankings for key non-branded disease terms
- •
Engagement rate (e.g., time on page, downloads) with educational content
- Initiative:
Develop a 'Science of Possibility' Thought Leadership Platform
Business Impact:High
Market Opportunity:Leverages existing brand equity in innovation to build immediate trust and credibility in new therapeutic areas, differentiating Vertex from competitors on scientific rigor.
Success Metrics
- •
Media mentions and backlinks to the platform
- •
Video views and engagement with scientist profiles
- •
Growth in branded search queries related to Vertex's research
- Initiative:
Execute Targeted HCP Digital Education Campaigns
Business Impact:Medium
Market Opportunity:Directly influences the primary decision-makers (prescribers) for new therapies, accelerating market adoption and revenue growth while optimizing sales force efforts.
Success Metrics
- •
HCP registration and engagement within professional portals
- •
Click-through rates from targeted ads on medical networks
- •
Attribution of digital touchpoints to prescription lift (where measurable)
Leverage the immense brand authority and reputation for scientific excellence earned in Cystic Fibrosis to position Vertex as the proven innovator capable of solving intractable challenges in new therapeutic areas. The overarching strategy is to pivot from being 'the CF company' to being 'the company that delivers transformative science for serious diseases,' using the digital presence to provide the evidence and narrative for this evolution.
Competitive Advantage Opportunities
- •
Showcase the depth of scientific R&D by profiling the scientists and the discovery process, creating a competitive advantage based on transparency and expertise.
- •
Utilize superior financial resources to fund high-impact, direct-to-patient digital awareness campaigns (e.g., celebrity partnerships) to build brand recognition for new drugs faster than smaller biotech rivals.
- •
Create the definitive online resource for the science behind CRISPR and NaV1.8 inhibition, establishing an educational moat that competitors cannot easily cross.
Vertex Pharmaceuticals has masterfully leveraged its dominant position in the Cystic Fibrosis market to build a powerful brand synonymous with breakthrough scientific innovation. Its current digital presence effectively communicates this core identity through a clean, professional interface that caters to its primary audiences: investors, media, healthcare professionals (HCPs), and prospective talent.
The strategic imperative for Vertex is now to transition its brand authority and market perception from a CF-focused leader to a diversified biotechnology powerhouse. The recent approvals of CASGEVY (gene therapy for sickle cell disease) and JOURNAVX (a first-in-class non-opioid pain treatment) represent monumental business opportunities but also thrust Vertex into crowded and highly competitive markets for the first time.
Strategic Challenges & Opportunities:
-
Brand Extension: The primary challenge is to extend its hard-won credibility to new disease states. While the 'Science of Possibility' tagline is an excellent thematic bridge, the digital strategy must now build deep, authoritative content ecosystems for each new therapeutic area. This is not merely a marketing task but a core business strategy to establish immediate trust with new communities of patients and prescribers.
-
Competitive Differentiation: In the gene therapy space, Vertex faces a direct competitor in Bluebird Bio. Its digital strategy must clearly articulate the unique advantages of its CRISPR-based approach. In the acute pain market, the challenge is even greater, requiring a powerful narrative to position JOURNAVX against inexpensive, established opioids. The digital presence must become the primary channel for educating the market on this paradigm shift in pain management.
-
Audience Acquisition: Vertex must move beyond attracting an audience that already knows its name. The future growth depends on capturing individuals searching for solutions to problems—'sickle cell cure,' 'non-addictive pain relief'—and leading them to Vertex's science. This requires a sophisticated, non-branded content strategy that positions Vertex as the definitive educational resource.
Recommendations:
The strategic focus should be a deliberate and well-funded expansion of the digital presence into distinct 'Centers of Excellence' for each major new therapeutic area. These hubs should serve all audiences, from foundational patient education to in-depth clinical data for HCPs. By creating the most comprehensive and scientifically rigorous digital resources, Vertex can replicate its leadership model from CF. This initiative, combined with a thought leadership platform that spotlights its world-class scientists, will transform its digital presence from a corporate brochure into a strategic asset that actively drives market expansion, builds competitive moats, and accelerates the adoption of its next generation of transformative medicines.
Strategic Priorities
Strategic Priorities
- Title:
Establish and Scale New Commercial Franchises Beyond Cystic Fibrosis
Business Rationale:The company's primary strategic risk is its heavy revenue concentration on the Cystic Fibrosis (CF) franchise. Successfully launching and scaling CASGEVY (gene therapy) and JOURNAVX (pain) in their respective unique markets is critical to de-risk the business and prove the 'serial innovation' model to investors and the market.
Strategic Impact:This transforms Vertex from a single-franchise company into a diversified, multi-franchise biopharmaceutical leader, significantly increasing its total addressable market and creating a more resilient long-term business structure.
Success Metrics
- •
Annual revenue from non-CF products (Target: >$2B within 3 years)
- •
Market share capture for CASGEVY vs. key competitors
- •
Patient uptake and prescription volume for JOURNAVX in the acute pain market
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Market Position
- Title:
Pioneer and Implement a Global Value-Based Access Model for Curative Therapies
Business Rationale:The ultra-high price point of one-time curative therapies like CASGEVY creates significant market access challenges with payers and health systems. Proactively developing and deploying innovative reimbursement models (e.g., outcomes-based annuities) is essential to overcome budget barriers, accelerate patient uptake, and justify the premium pricing strategy.
Strategic Impact:Establishes Vertex as an industry leader in commercializing curative therapies, creating a replicable framework for its future pipeline (e.g., Type 1 Diabetes). This builds a competitive moat based on market access expertise, not just science.
Success Metrics
- •
Reduction in average time-to-reimbursement approval in key markets
- •
Number of value-based or innovative payment agreements signed with major payers
- •
Rate of successful prior authorizations for CASGEVY
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Revenue Model
- Title:
Build a Scalable, World-Class Cell & Gene Therapy Operations Platform
Business Rationale:The analysis identifies manufacturing complexity and supply chain logistics as a primary bottleneck for cell and gene therapies. Investing in a dedicated, scalable operations platform is not just a technical need but a strategic imperative to meet patient demand for CASGEVY and future cell therapies, ensuring that commercial success is not limited by operational constraints.
Strategic Impact:Creates a profound and durable competitive advantage in the next generation of medicine. An efficient, in-house manufacturing and logistics capability becomes a core asset that is difficult for competitors to replicate and enables faster delivery of complex therapies.
Success Metrics
- •
Reduced 'vein-to-vein' time for CASGEVY treatment
- •
Decreased Cost of Goods Sold (COGS) per patient treatment
- •
Increased number of patients successfully treated per quarter
Priority Level:HIGH
Timeline:Long-term Vision (12+ months)
Category:Operations
- Title:
Accelerate 'Pillar' Pipeline Programs to Secure the Next Growth Wave
Business Rationale:Long-term growth depends on the success of the next wave of innovation beyond the current launches. The analysis highlights the transformative potential of the inaxaplin (kidney disease) and Type 1 Diabetes programs. Strategically accelerating these late-stage assets through focused resource allocation and aggressive trial recruitment is critical to solidifying the future revenue pipeline.
Strategic Impact:De-risks future revenue cliffs and maintains the company's growth narrative and high valuation. A successful outcome in either of these large markets would create another dominant franchise on the scale of Cystic Fibrosis.
Success Metrics
- •
Time to complete enrollment in pivotal Phase 3 trials for inaxaplin and zimislecel
- •
Achievement of key regulatory milestones (e.g., Fast Track, Accelerated Approval)
- •
Updated long-term revenue projections based on pipeline progress
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Revenue Model
- Title:
Execute a Strategic M&A Program to Acquire Next-Generation Platforms and Assets
Business Rationale:While internal R&D is strong, it is also high-risk. Utilizing the significant cash flow from the CF franchise for strategic, bolt-on acquisitions (like the Alpine deal) is a crucial lever to de-risk the pipeline, acquire new technologies (e.g., in-vivo gene editing), and accelerate entry into adjacent therapeutic areas.
Strategic Impact:Transforms the company's ability to adapt and lead in a rapidly evolving scientific landscape. A disciplined M&A strategy allows Vertex to systematically buy, not just build, future growth, ensuring a more robust and diverse long-term portfolio.
Success Metrics
- •
Number of targeted, high-value assets acquired or licensed per year
- •
Contribution of acquired assets to the mid-to-late stage pipeline
- •
Speed of integration and synergy realization from acquired companies
Priority Level:HIGH
Timeline:Long-term Vision (12+ months)
Category:Partnerships
Vertex must execute a pivotal transition from its dominant, single-franchise position in Cystic Fibrosis to a diversified, multi-franchise leader. This requires flawlessly commercializing its new, distinct therapies in pain and gene therapy while aggressively advancing its high-potential late-stage pipeline to secure the next wave of transformative growth.
The core competitive advantage to build is 'serial innovation mastery': the proven, repeatable ability to dominate a complex disease area, leverage the resulting financial strength to pioneer a completely new therapeutic modality or disease category, and systematically replicate this cycle to solve intractable medical challenges.
The primary growth catalyst will be the successful commercial diversification away from CF dependency. Achieving blockbuster status for at least one non-CF therapy will validate the scalability of Vertex's R&D and commercialization engine, unlocking the next tier of enterprise value and market leadership.