eScore
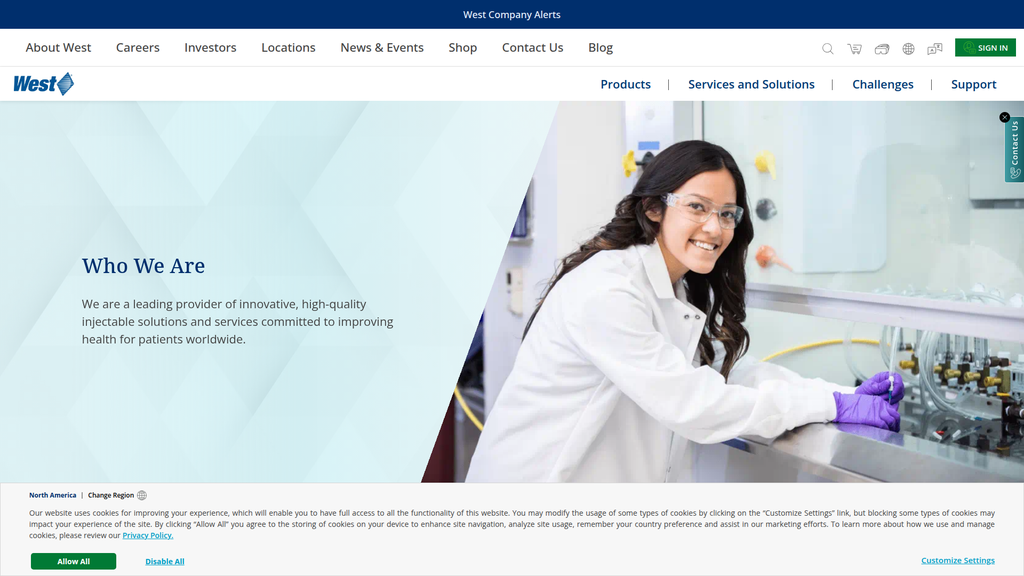
westpharma.comThe eScore is a comprehensive evaluation of a business's online presence and effectiveness. It analyzes multiple factors including digital presence, brand communication, conversion optimization, and competitive advantage.
West Pharma has a strong digital presence centered around its authoritative 'Knowledge Center,' which aligns well with the search intent of its technical B2B audience. The company demonstrates thought leadership on complex regulatory topics, reinforcing its brand authority. However, the content is heavily weighted towards bottom-of-funnel consideration, lacking broader, problem-oriented content to capture prospects earlier in their journey. While its global presence is clear, the digital experience lacks deep localization, and there is a missed opportunity to be more visible on high-growth trend keywords like 'GLP-1 drug delivery' where competitors are more aggressive.
The 'Knowledge Center' is a significant asset, establishing high content authority and serving the technical search intent of its core engineering and scientific audience effectively.
Develop a dedicated content hub for early-stage, problem-aware prospects (e.g., 'Solving Biologic Drug Stability'), and create targeted SEO campaigns around high-growth therapeutic areas to capture future market share, not just serve the existing base.
West's brand communication is highly effective, consistent, and well-aligned with its target market. The core message of being a trusted partner 'By Your Side for a Healthier World' effectively blends rational appeals to security (100-year history, quality) with emotional appeals to patient well-being. Messaging is clearly segmented for different audiences, from large pharma to emerging developers, addressing specific pain points like supply chain risk and regulatory compliance. The primary weakness is an over-reliance on generic calls-to-action ('Learn More') and a significant gap in using customer testimonials or case studies to substantiate its 'trusted partner' claim.
The messaging masterfully builds trust by anchoring its value proposition in longevity ('100 Years') and scale ('43 billion components'), which directly addresses the core risk-aversion of its pharmaceutical client base.
Incorporate direct customer proof, such as short case studies or partner testimonials, on key pages to bring the 'By Your Side' partnership message to life and validate claims with tangible success stories.
The website provides a functional and logical user experience with a clean information architecture and good mobile responsiveness. However, it suffers from a lack of persuasive design, which hinders conversion. A major friction point is the inconsistent and weak visual hierarchy of calls-to-action, with most being styled as low-prominence ghost buttons or links. The site also has accessibility gaps and areas of high cognitive load due to dense text blocks, which could be better optimized with visual aids to improve comprehension and guide users toward key actions.
The website's top-level navigation and information architecture are logical and clear, allowing users with specific intent (e.g., finding a product category) to navigate effectively.
Establish and consistently apply a clear visual CTA hierarchy: use solid, brand-color buttons for primary actions ('Contact Sales'), ghost buttons for secondary actions, and links for tertiary ones. This single change would significantly improve user guidance and conversion rates.
West Pharma's credibility is exceptionally high, built on a 100-year history and its status as an S&P 500 company. The website effectively showcases numerous trust signals, including its vast global scale, numerous industry awards, and thought leadership in regulatory compliance. Its business model inherently mitigates risk for customers by ensuring quality and navigating complex FDA/EMA regulations. While transparency in areas like pricing is low, which is typical for B2B manufacturing, the company's robust privacy policies and clear terms of sale further solidify its position as a highly credible and low-risk partner.
Leveraging its deep regulatory expertise as a strategic asset, particularly through its 'Regulatory Support' content, transforms compliance from a mere obligation into a powerful tool for building customer trust and demonstrating partnership.
While overall credibility is high, the absence of direct customer success evidence like case studies or testimonials on the website is a missed opportunity to add a powerful layer of third-party validation.
West Pharma's competitive advantage is formidable and highly sustainable, constituting a powerful economic moat. The primary advantage is the 'regulatory lock-in' created when its components are specified in a customer's Drug Master File, making switching suppliers prohibitively costly and risky. This is reinforced by a 100-year reputation for quality, a dominant market share of around 70%, and a global manufacturing footprint that is difficult to replicate. While the messaging around 'innovation' is identified as a minor weakness, the core advantages are deeply entrenched and sustainable.
Regulatory lock-in via the Drug Master File (DMF) creates exceptionally high switching costs, effectively making West an embedded partner for the entire lifecycle of a successful drug.
Proactively showcase innovation by creating a dedicated website section for 'Innovation in Action,' featuring new technologies, R&D breakthroughs, and forward-looking solutions to counter the perception of being just an established, reliable manufacturer.
The company has a highly scalable business model with extremely favorable unit economics and a strong track record of expanding margins with growth. West is perfectly positioned to capitalize on powerful market tailwinds, including the growth of biologics and GLP-1 therapies. Its global footprint and ongoing capital investments in capacity signal strong readiness for market expansion. The main constraints are the capital-intensive nature of manufacturing and the long lead times required to build and validate new facilities to meet surging demand.
West's business model has high operational leverage; once a component is designed into a major drug, it generates a long-term, high-margin revenue stream with minimal incremental sales cost, making growth highly profitable.
Develop more agile and flexible service models, such as a 'Biotech Fast-Track' program, to reduce friction and accelerate penetration into the high-growth emerging pharma and cell & gene therapy segments.
West Pharma's business model is exceptionally coherent and strategically focused. There is perfect alignment between its value proposition (de-risking drug development) and the primary needs of its target market (quality, reliability, compliance). Revenue streams are optimized towards high-margin proprietary products, and resource allocation is clearly focused on high-growth drivers like biologics. The model's timing is assessed as 'Excellent,' positioning the company to benefit directly from the most significant trends in the pharmaceutical industry.
The business model's alignment with the risk-averse, quality-focused needs of the injectable drug market is nearly perfect, making West an indispensable partner rather than a simple supplier.
Innovate the business model by monetizing its vast knowledge base more directly, such as by formalizing and expanding its analytical and regulatory consulting services into a distinct, high-margin revenue stream.
West Pharma exerts significant market power, underpinned by a dominant global market share of approximately 70% in its core segment. This leadership position, combined with the critical nature of its products and high switching costs, grants it strong pricing power. The company's 100-year history and deep integration with top-tier pharmaceutical clients allow it to influence industry standards and market direction. While formidable competitors exist, West's scale and regulatory entrenchment create a level of market power that is difficult to challenge.
Dominant market share and deep integration into the pharmaceutical supply chain provide significant leverage and pricing power, allowing the company to command premium prices for its high-value products.
Create a more formalized competitive intelligence function to monitor and counter the strategic messaging of competitors who are aggressively targeting high-growth niches like GLP-1s and digital health with their marketing.
Business Overview
Business Classification
B2B Manufacturing
Value-Added Services & B2B eCommerce
Healthcare
Sub Verticals
- •
Pharmaceutical Packaging
- •
Drug Delivery Systems
- •
Medical Device Components
Mature
Maturity Indicators
- •
Founded in 1923 (Over 100 years in operation).
- •
Publicly traded company (NYSE: WST) and S&P 500 component.
- •
Large-scale global operations: ~10,000 employees across ~50 sites.
- •
Annual shipment of over 43 billion components.
- •
Deeply integrated into the supply chain of the world's top pharmaceutical and biotech companies.
Enterprise
Steady
Revenue Model
Primary Revenue Streams
- Stream Name:
Proprietary Products Sales
Description:Sale of technologically advanced, high-quality injectable drug containment and delivery system components like stoppers, seals, plungers, and self-injection devices. This is the core business, representing approximately 80% of total revenue.
Estimated Importance:Primary
Customer Segment:Large Pharmaceutical & Biotech Companies
Estimated Margin:High
- Stream Name:
Contract-Manufactured Products
Description:Design, manufacture, and automated assembly of complex devices based on customers' intellectual property, such as insulin auto-injectors. This segment represents about 20% of revenue.
Estimated Importance:Secondary
Customer Segment:Pharmaceutical, Diagnostic & Medical Device Companies
Estimated Margin:Medium
- Stream Name:
Integrated Solutions & Services
Description:Value-added services including analytical laboratory testing, regulatory support, and other solutions that complement their product offerings, aimed at de-risking and accelerating drug development for clients.
Estimated Importance:Tertiary
Customer Segment:Emerging Biotech & Pharmaceutical R&D
Estimated Margin:Medium
- Stream Name:
Online Store Sales
Description:Direct-to-customer eCommerce channel offering ready-to-use products in smaller quantities, catering to R&D, clinical trials, and smaller-scale production needs.
Estimated Importance:Tertiary
Customer Segment:Emerging Drug Developers & Research Institutions
Estimated Margin:Medium
Recurring Revenue Components
Long-term supply agreements for commercialized drugs.
Regulatory 'lock-in' via Drug Master Files (DMFs), creating high switching costs and revenue streams that last the lifecycle of a drug.
Pricing Strategy
Value-Based & Contractual
Premium
Opaque
Pricing Psychology
Quality Signaling
Risk Reduction Premium
Monetization Assessment
Strengths
- •
Extremely high customer switching costs due to regulatory lock-in (Drug Master File).
- •
Pricing power derived from the critical nature and low relative cost of their components within the total drug price.
- •
Strong, recurring revenue from long-term contracts with blue-chip pharma clients.
Weaknesses
- •
Revenue is dependent on the R&D success and commercial lifecycle of their clients' drugs.
- •
Susceptible to broad pharmaceutical industry trends, such as patent cliffs or shifts in therapeutic focus.
- •
Recent performance shows sensitivity to post-pandemic inventory destocking and macroeconomic factors.
Opportunities
- •
Explosive growth in biologics and injectable therapies, particularly GLP-1 agonists for diabetes/obesity, requires advanced containment and self-injection systems.
- •
Increasing outsourcing of non-core activities by pharma companies, including high-value services like sterilization and packaging development.
- •
Expansion of integrated services to become a more indispensable partner, especially for emerging biotech firms.
Threats
- •
Competition from specialized players like Datwyler, AptarGroup, and Gerresheimer.
- •
Development of alternative drug delivery technologies (e.g., oral biologics) that could reduce demand for injectables.
- •
Increased scrutiny from regulators on packaging materials and potential for new, more stringent requirements.
Market Positioning
Quality and Reliability Leadership
Dominant (estimated ~70% of the global elastomeric components market for injectable drugs).
Target Segments
- Segment Name:
Global Pharmaceutical & Biotechnology Companies
Description:The world's largest and most established drug manufacturers who require a reliable, scaled, and globally compliant supply chain for their blockbuster injectable drugs.
Demographic Factors
- •
Large multinational corporations
- •
Publicly traded
- •
Extensive R&D and manufacturing footprints
Psychographic Factors
- •
Extremely risk-averse
- •
Value supply chain security and regulatory compliance above all else
- •
Focus on long-term partnerships
Behavioral Factors
- •
Long sales cycles
- •
Deep integration with suppliers
- •
High-volume, long-term purchasing contracts
Pain Points
- •
Regulatory approval delays
- •
Supply chain disruptions
- •
Maintaining drug product sterility and stability
- •
Product recalls due to container closure integrity failure
Fit Assessment:Excellent
Segment Potential:Medium
- Segment Name:
Emerging & Niche Drug Developers
Description:Smaller, often venture-backed, biotech and pharmaceutical companies focused on developing novel therapies, including biologics, cell, and gene therapies.
Demographic Factors
- •
Start-up to SMB size
- •
Often pre-revenue or in clinical trial stages
- •
Geographically concentrated in biotech hubs
Psychographic Factors
- •
Highly focused on speed-to-market
- •
Resource-constrained (both capital and personnel)
- •
Seek external expertise to navigate complex development pathways
Behavioral Factors
- •
Require smaller product quantities for R&D and clinical phases
- •
Value technical and regulatory support services
- •
Decision-making is faster but more price-sensitive
Pain Points
- •
Lack of in-house packaging and regulatory expertise
- •
Difficulty securing reliable supply for small-batch production
- •
Navigating complex global regulatory requirements
- •
High cost of failure in clinical trials
Fit Assessment:Good
Segment Potential:High
- Segment Name:
Generic & Biosimilar Manufacturers
Description:Companies focused on producing off-patent injectable drugs, where cost-efficiency and speed-to-market are paramount.
Demographic Factors
Varies from large multinational divisions to specialized regional players
Psychographic Factors
Highly cost-conscious
Focus on operational efficiency and proven solutions
Behavioral Factors
Seek established, approved components to minimize regulatory hurdles
Procurement is often price-driven
Pain Points
- •
Tight profit margins
- •
Need for rapid regulatory approval post-patent expiry
- •
Ensuring component compatibility with established drug formulations
Fit Assessment:Good
Segment Potential:Medium
Market Differentiation
- Factor:
Regulatory Lock-In (Drug Master File)
Strength:Strong
Sustainability:Sustainable
- Factor:
100+ Year Reputation for Quality and Reliability
Strength:Strong
Sustainability:Sustainable
- Factor:
Global Manufacturing & Supply Chain Footprint
Strength:Strong
Sustainability:Sustainable
- Factor:
Deep Scientific and Technical Expertise
Strength:Moderate
Sustainability:Sustainable
Value Proposition
We are the trusted global partner for ensuring the safe, effective containment and delivery of injectable medicines, mitigating risk and accelerating our customers' path to market through unparalleled quality, scientific expertise, and supply chain reliability.
Excellent
Key Benefits
- Benefit:
Mitigation of Regulatory and Development Risk
Importance:Critical
Differentiation:Unique
Proof Elements
- •
Decades of successful drug application filings using West components.
- •
Extensive proprietary data on container-drug interactions.
- •
Dedicated regulatory affairs support and analytical services.
- Benefit:
Ensuring Patient Safety and Drug Efficacy
Importance:Critical
Differentiation:Somewhat unique
Proof Elements
- •
Commitment to 'Leadership in Quality' value.
- •
Production of billions of components annually with rigorous quality control.
- •
Recognized as 'Best Primary Packaging Specialist' by industry awards.
- Benefit:
Global Supply Chain Security
Importance:Important
Differentiation:Somewhat unique
Proof Elements
- •
Manufacturing presence across North America, Europe, and Asia.
- •
Large scale with over 50 global sites.
- •
Ability to serve the world's largest pharmaceutical companies.
Unique Selling Points
- Usp:
Regulatory Embeddedness: Once a West component is specified in a drug's regulatory filing (DMF), it becomes the validated standard for that product's lifecycle, creating a powerful, long-term moat.
Sustainability:Long-term
Defensibility:Strong
- Usp:
Integrated System Approach: Offering a comprehensive portfolio from primary containment (vials, stoppers) to delivery systems (syringes, auto-injectors) and supporting analytical services, providing a single-partner solution.
Sustainability:Long-term
Defensibility:Moderate
Customer Problems Solved
- Problem:
Protecting sensitive, high-value biologic drugs from degradation due to interaction with packaging materials.
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Navigating the complex and costly regulatory approval process for drug container and closure systems.
Severity:Critical
Solution Effectiveness:Complete
- Problem:
Ensuring a sterile, global, and reliable supply of critical components for life-saving medicines.
Severity:Major
Solution Effectiveness:Complete
Value Alignment Assessment
High
The business model is perfectly aligned with the needs of the growing injectable drug market, which prioritizes safety, quality, and regulatory compliance. The secular trend towards biologics and self-administered therapies directly plays to West's strengths in high-value components and delivery systems.
High
The value proposition directly addresses the primary pain points of pharmaceutical companies: de-risking development, ensuring product integrity, and securing the supply chain. This focus has made West an indispensable partner rather than a mere supplier.
Strategic Assessment
Business Model Canvas
Key Partners
- •
Pharmaceutical companies (e.g., Pfizer, Merck, J&J)
- •
Biotechnology firms
- •
Generic and biosimilar drug manufacturers
- •
Contract Development and Manufacturing Organizations (CDMOs)
- •
Medical device companies
Key Activities
- •
Research & Development in material science and engineering
- •
High-precision, large-scale manufacturing
- •
Stringent Quality Assurance and Control
- •
Regulatory Affairs and Compliance Management
- •
Global Supply Chain Logistics
Key Resources
- •
Intellectual Property (patents, proprietary material formulations)
- •
Global network of cGMP manufacturing facilities
- •
Decades of regulatory filing data (Drug Master Files)
- •
Skilled workforce of scientists, engineers, and regulatory experts
- •
Strong brand reputation and customer relationships
Cost Structure
- •
Manufacturing costs (raw materials, energy, labor)
- •
Research & Development investment
- •
Capital expenditures for facility expansion and technology upgrades
- •
Selling, General & Administrative (SG&A) expenses
- •
Quality control and regulatory compliance costs
Swot Analysis
Strengths
- •
Dominant market share and brand leadership built over 100 years.
- •
Extremely high barriers to entry due to regulatory requirements and customer switching costs.
- •
Deeply integrated, long-term relationships with a blue-chip customer base.
- •
Strong and consistent cash flow generation.
- •
Global manufacturing footprint providing supply chain resilience.
Weaknesses
- •
High capital intensity required for manufacturing and R&D.
- •
Growth is largely dependent on the broader pharmaceutical market's R&D pipeline and success.
- •
Complex global operations can be exposed to geopolitical and logistical risks.
Opportunities
- •
Continued growth in the biologics, cell/gene therapy, and biosimilar markets.
- •
Rapidly expanding demand for self-injection devices driven by trends like GLP-1 drugs for chronic disease management.
- •
Expansion of high-margin analytical and regulatory services, moving further up the value chain.
- •
Strategic acquisitions to enter adjacent technology areas (e.g., smart packaging, data services).
Threats
- •
Innovation in oral or alternative drug delivery methods that could reduce the need for injectables.
- •
Intensifying competition from nimble, specialized players in high-growth niches.
- •
Global economic downturns impacting pharmaceutical R&D spending and new drug launches.
- •
Supply chain volatility and rising costs of raw materials and energy.
Recommendations
Priority Improvements
- Area:
Digital Customer Experience
Recommendation:Accelerate the development of digital platforms, like the Knowledge Center, to provide self-service access to technical data, regulatory documentation, and modeling tools. This is crucial for attracting and retaining fast-moving emerging biotech customers.
Expected Impact:Medium
- Area:
Service Model Innovation
Recommendation:Develop and formalize a 'Biotech-in-a-Box' service offering that bundles small-scale components with a comprehensive package of analytical testing and regulatory filing support, potentially on a milestone-based payment model.
Expected Impact:High
- Area:
Supply Chain for Next-Generation Therapies
Recommendation:Proactively invest in manufacturing capabilities and material science R&D specifically for cell and gene therapies, which have unique and highly demanding containment requirements.
Expected Impact:High
Business Model Innovation
Launch a dedicated corporate venture arm to invest in and partner with early-stage companies developing disruptive drug delivery technologies (e.g., smart injectors, large-volume wearables).
Develop a data-as-a-service (DaaS) platform that leverages West's vast repository of drug-container interaction data to help clients predict stability and select optimal packaging, accelerating their development timelines.
Revenue Diversification
- •
Expand the standalone analytical services business to capture revenue from clients not yet purchasing physical products.
- •
Acquire a specialized consultancy firm to build out a premium regulatory strategy and advisory service.
- •
Explore licensing proprietary material science and coating technologies for applications in other regulated, high-value industries beyond pharmaceuticals.
West Pharmaceutical Services exemplifies a mature, highly defensible business model built on a foundation of quality, regulatory expertise, and deep customer integration. Its primary strength lies in the formidable barriers to entry created by the regulatory 'lock-in' of its components in drug filings, which translates into sticky, long-term, and high-margin revenue streams. The company is correctly positioned as the premium, reliable choice in a risk-averse industry.
The key strategic imperative for West is to evolve from being a component manufacturer to a full-fledged solutions partner, particularly for the high-growth emerging biotech segment. While its core business with large pharma is secure and provides a stable cash cow, future growth will be driven by its ability to help smaller, resource-constrained companies navigate the complexities of drug development. The expansion into integrated services and the development of digital tools are critical steps in this evolution.
Future business model transformation should focus on capturing more value from its immense knowledge base. West doesn't just sell components; it sells certainty and speed-to-market. Monetizing this expertise more directly through data services, advanced consulting, and innovative partnership models will be key to sustaining its growth trajectory. The secular tailwinds from biologics and self-administered chronic care therapies (like GLP-1s) provide a significant runway for growth, but maintaining its leadership will require continuous innovation in both products and business models to preempt competition and address the novel challenges of next-generation medicines.
Competitors
Competitive Landscape
Mature
Oligopoly
Barriers To Entry
- Barrier:
Stringent Regulatory Hurdles
Impact:High
- Barrier:
High Capital Investment for Manufacturing
Impact:High
- Barrier:
Intellectual Property and Patents
Impact:High
- Barrier:
Established Customer Relationships and Trust
Impact:High
- Barrier:
Complex Global Supply Chains
Impact:Medium
Industry Trends
- Trend:
Growth of Biologics and Biosimilars
Impact On Business:Drives demand for high-value, specialized containment systems (e.g., polymer vials, advanced stoppers) to ensure drug stability and safety.
Timeline:Immediate
- Trend:
Shift Towards Self-Administration and Home Care
Impact On Business:Increases demand for patient-centric delivery systems like auto-injectors and wearable devices.
Timeline:Immediate
- Trend:
Increasing Focus on Sustainability
Impact On Business:Requires investment in R&D for recyclable, biodegradable, and lower-footprint materials and designs.
Timeline:Near-term
- Trend:
Smart Packaging and Digitalization
Impact On Business:Creates opportunities for value-added services like track-and-trace, patient adherence monitoring, and data integration.
Timeline:Near-term
- Trend:
Demand for GLP-1 Therapies
Impact On Business:Explosive growth in diabetes and obesity treatments is a major revenue driver for companies providing high-quality components for these injectable drugs.
Timeline:Immediate
Direct Competitors
- →
Gerresheimer AG
Market Share Estimate:Significant
Target Audience Overlap:High
Competitive Positioning:Positions as a broad-range provider of solutions in pharma and healthcare, with strong expertise in glass and plastic primary packaging and drug delivery devices.
Strengths
- •
Strong heritage and expertise in glass manufacturing (vials, syringes).
- •
Broad portfolio spanning glass, plastic, and drug delivery systems.
- •
Global manufacturing footprint.
Weaknesses
- •
May be perceived as less specialized in high-performance elastomers compared to West.
- •
Recent revenue decreases and strategic reviews could indicate internal challenges.
- •
Less emphasis on integrated containment and delivery systems in their marketing compared to West.
Differentiators
Deep vertical integration in glass production.
Strong focus on both pharmaceutical and cosmetic packaging markets.
- →
SCHOTT Pharma
Market Share Estimate:Significant
Target Audience Overlap:High
Competitive Positioning:Positions as a specialist in glass and polymer drug containment and delivery systems, emphasizing material science and innovation.
Strengths
- •
World-leading expertise in pharmaceutical glass (Type I Borosilicate Glass).
- •
Strong reputation for quality and reliability.
- •
Innovation in polymer syringes and vials suitable for sensitive biologics.
- •
Offers comprehensive pharma services including analytics and regulatory support.
Weaknesses
- •
Less focused on elastomer components (stoppers, plungers) which are a core strength of West.
- •
Primarily focused on the container, less on the complete integrated system including delivery devices.
- •
Newer as a publicly traded, standalone entity, having spun off from Schott AG.
Differentiators
Unmatched expertise and brand equity in pharmaceutical glass.
Focus on material science as a core value proposition.
- →
AptarGroup, Inc. (Pharma Division)
Market Share Estimate:Significant
Target Audience Overlap:Medium
Competitive Positioning:Positions as a leader in drug delivery systems, particularly for nasal, pulmonary, and injectable routes, as well as active packaging solutions.
Strengths
- •
Market leader in dispensing systems like nasal sprays and metered-dose inhalers.
- •
Strong and resilient pharma division driving corporate growth.
- •
Innovative portfolio in connected devices and active material science.
- •
Diversified business across pharma, beauty, and food & beverage reduces risk.
Weaknesses
- •
Less focused on primary containment (vials, stoppers) compared to West.
- •
Injectables business is a smaller part of their overall pharma segment compared to other delivery routes.
- •
Reliance on single-source suppliers for some materials is a stated operational risk.
Differentiators
Leadership in a wide array of dispensing technologies beyond injectables.
Strong focus on consumer-facing applications (nasal, ophthalmic) which informs their patient-centric design.
- →
Datwyler Holding Inc.
Market Share Estimate:Growing
Target Audience Overlap:High
Competitive Positioning:Positions as a key player for system-critical elastomer components, emphasizing advanced formulations, coatings, and quality for injectable drug packaging.
Strengths
- •
Deep expertise in elastomer and rubber formulations for stoppers and plungers.
- •
Strong focus on high-quality solutions for sensitive biologics and biosimilars.
- •
Offers advanced coatings and treatments to ensure drug compatibility and safety.
Weaknesses
- •
Less known for integrated delivery systems (e.g., auto-injectors) compared to West.
- •
Brand recognition may be lower than West or SCHOTT in the broader market.
- •
More focused on components rather than complete system solutions.
Differentiators
Specialization and deep scientific expertise in elastomer components.
Emphasis on customized solutions and close collaboration with pharma clients.
- →
Becton, Dickinson and Company (BD)
Market Share Estimate:Significant
Target Audience Overlap:Medium
Competitive Positioning:A global medical technology giant with a very strong position in prefillable syringes and safety injection devices, competing directly with West in the drug delivery space.
Strengths
- •
Dominant market position in syringes, needles, and catheters.
- •
Extensive global reach and deep relationships with hospitals and clinics.
- •
Broad portfolio of medical devices beyond drug delivery creates cross-selling opportunities.
- •
Strong brand recognition and reputation in the healthcare industry.
Weaknesses
- •
Less focused on the primary containment components like stoppers and seals, which they often source.
- •
Their business model is broader, so they may be less specialized in the niche of containment science than West.
- •
Competition is vast, spanning numerous healthcare segments.
Differentiators
End-to-end solutions from drug delivery to diagnostics and clinical research.
Strong focus on injection safety for healthcare workers.
Indirect Competitors
- →
Catalent, Inc.
Description:A leading Contract Development and Manufacturing Organization (CDMO) that provides drug development, delivery, and supply services. They handle fill-finish operations and can influence packaging decisions.
Threat Level:Medium
Potential For Direct Competition:Increasingly, large CDMOs are vertically integrating packaging solutions as part of their end-to-end service offering, which could shift the customer relationship away from component suppliers like West.
- →
Nemera
Description:Designs and manufactures drug delivery devices, including inhalers, spray pumps, and auto-injectors. While they are a partner to pharma companies, they compete with West's self-injection device portfolio.
Threat Level:Medium
Potential For Direct Competition:They are already a direct competitor in the device space. The threat would increase if they backward integrate into elastomer or vial production.
- →
Companies developing alternative delivery technologies
Description:Firms specializing in non-injectable delivery for biologics, such as oral, transdermal (patches), or pulmonary (advanced inhalation) methods. Success in these areas could reduce the total addressable market for injectables.
Threat Level:Low
Potential For Direct Competition:Low, as their focus is on fundamentally different technologies. However, they are competing for the same drug pipelines at an early stage.
Competitive Advantage Analysis
Sustainable Advantages
- Advantage:
Integrated System Approach ('By Your Side')
Sustainability Assessment:Highly sustainable. The combination of high-quality components, delivery devices, and analytical services creates a sticky ecosystem that is difficult for competitors to replicate.
Competitor Replication Difficulty:Hard
- Advantage:
Regulatory Expertise and Reputation for Quality
Sustainability Assessment:Highly sustainable. Decades of experience navigating global regulatory bodies (FDA, EMA) and a reputation for minimizing risks for clients create a powerful moat.
Competitor Replication Difficulty:Hard
- Advantage:
Deep, Long-Term Customer Relationships
Sustainability Assessment:Sustainable. West is embedded in the development and lifecycle of its customers' blockbuster drugs, making switching suppliers costly and risky for the pharmaceutical company.
Competitor Replication Difficulty:Hard
- Advantage:
High-Value Proprietary Products (HVP)
Sustainability Assessment:Highly sustainable. Products like FluroTec® films and Daikyo Crystal Zenith® polymers offer performance benefits that are critical for sensitive biologics, commanding premium pricing and creating high switching costs.
Competitor Replication Difficulty:Medium
Temporary Advantages
{'advantage': 'Specific Product Patents', 'estimated_duration': '5-15 years (depending on patent lifecycle). Competitors will engineer around expired patents.'}
Disadvantages
- Disadvantage:
Potential for Higher Price Point
Impact:Minor
Addressability:Moderately
- Disadvantage:
Perceived Complexity for Small/Emerging Pharma
Impact:Minor
Addressability:Easily
Strategic Recommendations
Quick Wins
- Recommendation:
Expand and heavily market the 'Online Store' with more products and resources specifically for early-stage and small-batch customers.
Expected Impact:Medium
Implementation Difficulty:Easy
- Recommendation:
Launch a targeted digital marketing campaign highlighting West's role in the successful delivery of GLP-1 drugs to capitalize on current market trends.
Expected Impact:High
Implementation Difficulty:Moderate
- Recommendation:
Create and promote thought leadership content comparing the total cost of ownership (TCO) of integrated, high-quality systems vs. sourcing low-cost individual components, emphasizing risk reduction.
Expected Impact:Medium
Implementation Difficulty:Moderate
Medium Term Strategies
- Recommendation:
Develop and launch a comprehensive portfolio of containment and delivery solutions specifically designed for cell and gene therapies.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Invest in next-generation sustainable materials, aiming for a fully recyclable or biodegradable integrated injection system.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Expand the 'Services' offering to include more advanced regulatory consulting and fill-finish process optimization to further embed West within customer operations.
Expected Impact:Medium
Implementation Difficulty:Moderate
Long Term Strategies
- Recommendation:
Acquire or partner with a technology firm specializing in smart packaging (NFC/RFID/sensors) to build a leading data-driven drug delivery platform.
Expected Impact:High
Implementation Difficulty:Difficult
- Recommendation:
Establish a venture arm to invest in promising alternative drug delivery technologies, creating an option for future market shifts.
Expected Impact:Medium
Implementation Difficulty:Difficult
Solidify and message West's position as the premier, end-to-end 'De-Risking Partner' for the development and delivery of high-value, complex injectable medicines.
Differentiate on the basis of integrated system reliability, data-backed quality, and expert scientific/regulatory support, moving the conversation from component price to lifecycle value and patient safety.
Whitespace Opportunities
- Opportunity:
End-to-End Solutions for Cell & Gene Therapy
Competitive Gap:The unique requirements (e.g., cryogenic storage) for cell and gene therapies are not yet fully addressed by integrated, off-the-shelf containment and delivery systems from major players.
Feasibility:Medium
Potential Impact:High
- Opportunity:
Packaging-as-a-Service (PaaS) for Clinical Trials
Competitive Gap:Small and mid-size biotech firms lack the internal resources for packaging selection, testing, and regulatory documentation during clinical phases. No major competitor offers a fully serviced, streamlined solution for this segment.
Feasibility:High
Potential Impact:Medium
- Opportunity:
Sustainable Lifecycle Management
Competitive Gap:While competitors are focusing on recyclable materials, none offer a comprehensive service that includes material selection, lifecycle assessment, and a take-back/recycling program for used delivery devices.
Feasibility:Medium
Potential Impact:Medium
- Opportunity:
Data and Analytics Services from Smart Devices
Competitive Gap:While 'smart packaging' is an emerging trend, no competitor has a dominant platform that provides pharmaceutical companies with robust, real-world data on patient adherence and handling conditions from connected delivery devices.
Feasibility:Low
Potential Impact:High
West Pharmaceutical Services operates within a mature, oligopolistic market for injectable drug containment and delivery systems. The competitive landscape is defined by high barriers to entry, including stringent regulations, significant capital requirements, and the critical importance of quality and trust. West has successfully carved out a premium position by differentiating itself as an integrated solutions partner ('By Your Side'), combining high-quality components (stoppers, plungers), delivery devices (auto-injectors), and essential scientific and analytical services.
Its primary competitors—Gerresheimer, SCHOTT Pharma, AptarGroup, Datwyler, and Becton Dickinson—each present a formidable challenge but often with a different center of gravity. SCHOTT is the undisputed leader in glass science, Datwyler excels in elastomer components, Aptar leads in diverse dispensing technologies beyond injectables, Gerresheimer offers a broad portfolio with a glass and plastic focus, and BD dominates the clinical point-of-care with its vast range of syringes and needles. West's key sustainable advantage is its ability to seamlessly integrate these elements into a reliable, de-risked system for its pharmaceutical partners, a proposition that is particularly compelling for high-value biologic and GLP-1 drugs.
The primary industry trends, such as the rise of biologics, the shift to self-administration, and the push for sustainability, all play to West's strengths and strategic focus. However, to maintain its leadership, West must continue to innovate. Key whitespace opportunities exist in providing comprehensive solutions for the nascent cell and gene therapy market, developing a stronger service offering for small-scale clinical trials, and pioneering a truly sustainable lifecycle for its products. The long-term threat of alternative drug delivery methods remains low but requires monitoring. Strategic focus should be on deepening the integrated service model, expanding into adjacent high-science areas, and leveraging digital technologies to transform from a component supplier into a data-enabled healthcare partner.
Messaging
Message Architecture
Key Messages
- Message:
100 Years of Being By Your Side for a Healthier World™
Prominence:Primary
Clarity Score:High
Location:Homepage Hero Banner
- Message:
We are a leading provider of innovative, high-quality injectable solutions and services committed to improving health for patients worldwide.
Prominence:Primary
Clarity Score:High
Location:Homepage 'Who We Are' section
- Message:
As a trusted partner to established and emerging drug developers, we help ensure the safe, effective containment and delivery of life-saving and life-enhancing medicines for patients.
Prominence:Secondary
Clarity Score:High
Location:Homepage 'What We Do' section
- Message:
With approximately 10,000 team members across 50 sites worldwide, West helps support our customers with about 43 billion components and devices shipped annually.
Prominence:Tertiary
Clarity Score:High
Location:Homepage 'What We Do' section
The message hierarchy is logical and effective. It starts with the overarching brand promise of partnership and longevity ('By Your Side'), immediately followed by a clear and concise statement of what the company does and for whom. Supporting messages of scale, trust, and patient focus are layered underneath, providing depth and credibility.
Messaging is highly consistent across the homepage and 'About Us' section. Core concepts like 'high-quality,' 'trusted partner,' 'improving patient health,' and global leadership are repeated, reinforcing the brand's position. This repetition is effective for a B2B audience that values reliability and stability.
Brand Voice
Voice Attributes
- Attribute:
Professional & Authoritative
Strength:Strong
Examples
- •
We are a leading provider of innovative, high-quality injectable solutions...
- •
West is a leading global manufacturer in the design and production of technologically advanced, high-quality, integrated containment and delivery systems...
- •
Article Published in Pharmaceutical Manufacturer, 'EU GMP Annex 1: Tackling Compliance with Confidence...'
- Attribute:
Trustworthy & Reliable
Strength:Strong
Examples
- •
100 Years of Being By Your Side...
- •
As a trusted partner...
- •
You need reliable technical documentation...
- Attribute:
Patient-Centric & Empathetic
Strength:Moderate
Examples
- •
For us, things have always been personal; that’s why every product we develop has a patient's name on it.
- •
committed to improving health for patients worldwide.
- •
...life-saving and life-enhancing medicines for patients.
- Attribute:
Innovative
Strength:Weak
Examples
...technologically advanced, high-quality, integrated containment and delivery systems...
Tone Analysis
Corporate
Secondary Tones
- •
Reassuring
- •
Confident
- •
Formal
Tone Shifts
The tone shifts to be more personal and empathetic in the hero banner message: 'For us, things have always been personal; that’s why every product we develop has a patient's name on it.' This is a powerful, but brief, departure from the otherwise corporate tone.
Voice Consistency Rating
Good
Consistency Issues
The claim of being 'innovative' and 'technologically advanced' is stated but not strongly demonstrated through messaging on the homepage. It's a 'tell' rather than a 'show' and feels like a standard corporate adjective rather than a core voice attribute.
Value Proposition Assessment
To be the trusted, high-quality global leader in integrated containment and delivery systems for injectable medicines, ensuring the safety and efficacy of our partners' drugs and improving patient health.
Value Proposition Components
- Component:
Quality & Reliability
Clarity:Clear
Uniqueness:Common
- Component:
Partnership & Trust
Clarity:Clear
Uniqueness:Somewhat Unique
- Component:
Global Scale & Experience
Clarity:Clear
Uniqueness:Unique
- Component:
Patient Safety Focus
Clarity:Clear
Uniqueness:Somewhat Unique
West successfully differentiates itself through its emphasis on longevity ('100 Years') and partnership ('By Your Side'). While competitors also talk about quality, West frames it within a narrative of long-term reliability and customer collaboration. The massive scale (43 billion components) is a key differentiator that implies market leadership and supply chain security, which are critical concerns for their target audience. The emotional hook of 'every product has a patient's name on it' is a memorable and unique messaging angle in a typically sterile B2B industry.
The messaging positions West as the established, premium market leader. It's not trying to be the disruptor or the low-cost alternative. The focus is on de-risking the complex process of drug containment and delivery for major pharmaceutical and biotech companies. This positioning appeals to risk-averse buyers for whom quality, compliance, and supply chain stability are non-negotiable.
Audience Messaging
Target Personas
- Persona:
Procurement & Technical Teams at large Pharmaceutical/Biotechnology firms
Tailored Messages
- •
Leading provider of innovative, high-quality injectable solutions and services.
- •
43 billion components and devices shipped annually.
- •
Article Published in Pharmaceutical Manufacturer, 'EU GMP Annex 1: Tackling Compliance with Confidence...'
- •
Knowledge Center: Take an in-depth look at the science...
Effectiveness:Effective
- Persona:
Scientists & Formulation Developers at emerging drug companies
Tailored Messages
- •
As a trusted partner to established and emerging drug developers...
- •
Partnering with customers to align drug packaging, containment, and delivery products with services, solutions and support.
- •
Online Store: Need ready-to-use products in small packs?
Effectiveness:Somewhat Effective
Audience Pain Points Addressed
- •
Supply chain risk and reliability ('100 Years', '50 sites worldwide').
- •
Regulatory compliance ('EU GMP Annex 1' article).
- •
Ensuring drug safety and efficacy ('safe, effective containment and delivery').
- •
Accessing technical information and support ('Knowledge Center', 'My Account' portal).
- •
Sourcing small-batch components for R&D/early phase work ('Online Store').
Audience Aspirations Addressed
- •
Bringing life-saving and life-enhancing medicines to patients.
- •
Improving health for patients worldwide.
- •
Partnering with a market leader to ensure project success.
Persuasion Elements
Emotional Appeals
- Appeal Type:
Appeal to Purpose/Altruism
Effectiveness:High
Examples
- •
By Your Side for a Healthier World™
- •
...every product we develop has a patient's name on it.
- •
improve patient health.
- Appeal Type:
Appeal to Security/Safety
Effectiveness:High
Examples
- •
100 Years...
- •
trusted partner...
- •
ensure the safe, effective containment and delivery...
Social Proof Elements
- Proof Type:
Longevity/History
Impact:Strong
- Proof Type:
Market Scale
Impact:Strong
- Proof Type:
Industry Awards
Impact:Moderate
- Proof Type:
Expertise (Published Articles)
Impact:Moderate
Trust Indicators
- •
100-year history
- •
Global presence (50 sites, 10,000 team members)
- •
Vast scale of production (43 billion components annually)
- •
Explicit mention of 'trusted partner'
- •
Industry awards and publications
- •
Commitment to ESG and Corporate Giving
Scarcity Urgency Tactics
No itemsCalls To Action
Primary Ctas
- Text:
Learn More
Location:Multiple sections (100 Years, Products, Company Background, etc.)
Clarity:Somewhat Clear
- Text:
Register Here
Location:Announcements section
Clarity:Somewhat Clear
- Text:
Knowledge Center
Location:Main navigation/links
Clarity:Clear
- Text:
Online Store
Location:Main navigation/links
Clarity:Clear
- Text:
See Current Opportunities
Location:Careers section
Clarity:Clear
The CTAs are functional but lack persuasive power. 'Learn More' is generic and used excessively. 'Register Here' is an instruction, not a benefit; the value of registering is listed separately in bullet points, but the CTA itself could be stronger (e.g., 'Access Technical Resources'). The navigational CTAs ('Knowledge Center', 'Online Store') are clear and effective for users with specific intent. The overall CTA strategy could be improved by using more action-oriented and benefit-driven language.
Messaging Gaps Analysis
Critical Gaps
- •
Lack of tangible evidence for 'innovation'. While the word is used, there are no specific examples on the homepage of new technologies, R&D breakthroughs, or forward-looking solutions that support this claim.
- •
Absence of customer testimonials or case studies. While being a 'trusted partner' is a key message, there is no direct voice-of-customer to substantiate this relationship-focused claim.
- •
Quantifiable business outcomes. The messaging focuses on what West does, but not on the specific outcomes for customers (e.g., helping a partner reduce time-to-market, improving drug stability by X%, etc.).
Contradiction Points
No itemsUnderdeveloped Areas
Messaging for emerging biotech companies. The site mentions them as partners, but the overall messaging feels geared towards established, large pharmaceutical companies. Tailoring content to address the specific needs of smaller, more agile firms (speed, specialized support, navigating regulatory pathways for the first time) could be more effective.
The 'Services' aspect of their 'Services and Solutions' offering is not clearly articulated on the homepage. The focus is heavily on products and components.
Messaging Quality
Strengths
- •
Extremely clear market positioning as the established global leader.
- •
Excellent use of longevity ('100 Years') and scale ('43 billion components') to build trust and communicate reliability.
- •
The patient-centric message ('a patient's name on it') provides a powerful emotional connection point in a technical B2B market.
- •
Messaging is well-aligned with the core purchasing drivers in the pharmaceutical industry: quality, reliability, and regulatory compliance.
Weaknesses
- •
Over-reliance on corporate jargon ('leading provider', 'high-quality', 'technologically advanced') without sufficient supporting detail on the homepage.
- •
The 'innovation' message is asserted but not demonstrated, weakening its impact.
- •
Calls-to-action are generic and lack persuasive, benefit-oriented language.
Opportunities
- •
Feature a dedicated 'Innovation Hub' section on the homepage to showcase new technologies, materials science, or smart packaging solutions.
- •
Develop and feature concise case studies or partner stories that bring the 'By Your Side' message to life.
- •
Create more segmented messaging paths for different customer types (e.g., large pharma vs. emerging biotech) to address their distinct pain points more directly.
- •
Integrate sustainability messaging more prominently, as this is a growing trend and decision driver in pharmaceutical packaging.
Optimization Roadmap
Priority Improvements
- Area:
Value Proposition (Innovation)
Recommendation:Replace a generic 'What We Do' panel on the homepage with a dynamic 'Innovation in Action' section. Showcase 1-2 examples of advanced products or technologies with clear benefits (e.g., 'Our NovaPure® components reduce particulate risk by X%').
Expected Impact:High
- Area:
Persuasion & Trust
Recommendation:Incorporate a subtle customer logo bar or a short, powerful quote from a key partner on the homepage to provide direct social proof for the 'trusted partner' claim.
Expected Impact:High
- Area:
Calls to Action
Recommendation:Revise the primary CTAs to be more specific and benefit-driven. Change 'Register Here' to 'Access Technical Data Sheets & Tools'. Change generic 'Learn More' links to more descriptive text like 'Explore Our Vial Solutions' or 'Discover Our 100-Year History'.
Expected Impact:Medium
Quick Wins
Update the CTA text for the account registration to be more compelling.
Add a sub-headline to the 'Who We Are' section that quantifies their leadership, e.g., 'The Global Leader in Injectable Solutions, Trusted by the World's Top Pharmaceutical Companies.'
Long Term Recommendations
- •
Develop a content strategy around storytelling, creating detailed case studies and video testimonials that demonstrate the 'By Your Side' partnership model in practice.
- •
Build out dedicated landing pages and messaging streams for key audience segments like 'Emerging Biotech' and 'Established Global Pharma' to enhance audience-message fit.
- •
Conduct a competitive messaging audit to identify how rivals communicate innovation and partnership, ensuring West's differentiation remains sharp.
West Pharmaceutical Services' website employs a highly effective messaging strategy centered on trust, quality, and market leadership. The core messages are clear, consistent, and perfectly aligned with the priorities of its risk-averse B2B audience in the pharmaceutical and biotech industries. The brand voice is professional and authoritative, successfully positioning West as the reliable, established expert. Differentiation is achieved not just by claiming quality, but by anchoring it in a century of experience ('100 Years') and a unique, empathetic mission ('every product has a patient's name on it'). This creates a powerful blend of rational and emotional appeal.
The primary strength of the messaging is its ability to build immense trust. By emphasizing its vast scale, global footprint, and long history, West directly addresses the critical customer pain points of supply chain security and reliability. The architecture is logical, guiding the user from a high-level brand promise to more specific offerings.
However, there are significant opportunities for optimization. The messaging around 'innovation' is a major gap; it is claimed but not substantiated, leaving a key value proposition underdeveloped. Furthermore, the reliance on generic calls-to-action and the absence of direct customer proof (testimonials, case studies) represent missed opportunities to convert interest into engagement. To elevate its strategy, West should shift from simply stating its value to actively demonstrating it, particularly in the realm of technological advancement and customer success stories. By making its innovation tangible and allowing customers' voices to validate its partnership claims, West can create a more dynamic and persuasive digital presence that fully reflects its market leadership.
Growth Readiness
Growth Foundation
Product Market Fit
Strong
Evidence
- •
100-year history as a market leader in injectable drug containment and delivery systems.
- •
Dominant market share, estimated around 70% in its core elastomer components market.
- •
Trusted partner to the world's top pharmaceutical and biotechnology companies, deeply integrated into their supply chains.
- •
High-value product (HVP) portfolio, including Westar® and NovaPure®, represents over 70% of Proprietary Products segment sales, indicating strong demand for premium offerings.
- •
Extremely high switching costs for customers due to stringent regulatory requirements (e.g., Drug Master Files), which 'lock in' West's components for the life of a drug.
Improvement Areas
- •
Develop more agile service offerings for emerging biotech and cell & gene therapy startups with smaller batch sizes and faster development timelines.
- •
Expand the 'Online Store' functionality to better serve R&D and clinical trial material needs, reducing friction for smaller customers.
- •
Increase focus on sustainable materials and circular economy solutions to meet evolving ESG demands from large pharma partners.
Market Dynamics
The global injectable drug delivery market is projected to grow at a CAGR of 5.8% to 8.6% through 2034. The primary pharmaceutical packaging market is expected to grow at a CAGR of ~6-10%.
Mature
Market Trends
- Trend:
Growth in Biologics, Biosimilars, and GLP-1 Therapies
Business Impact:Drives significant demand for high-value, specialized containment and delivery systems (e.g., prefillable syringes, auto-injectors) to handle complex, sensitive molecules. This is a primary growth driver for West's HVP segment.
- Trend:
Rise of Cell & Gene Therapies (CGT)
Business Impact:Creates a new, high-growth market for novel packaging solutions that can handle cryogenic temperatures and maintain cell viability, presenting a major innovation opportunity.
- Trend:
Increasingly Stringent Regulatory Standards (e.g., EU GMP Annex 1)
Business Impact:Acts as a competitive advantage by raising the barrier to entry and driving a 'flight to quality,' benefiting established leaders like West with strong regulatory expertise and high-quality products.
- Trend:
Shift Towards Self-Administration and Home Care
Business Impact:Boosts demand for user-friendly self-injection devices and prefillable syringe systems, aligning perfectly with West's product portfolio and R&D focus.
- Trend:
Focus on Sustainability in Pharma Packaging
Business Impact:Requires investment in R&D for recyclable materials and circular solutions to maintain preferred supplier status with environmentally conscious global pharmaceutical companies.
Excellent. West is perfectly positioned at the intersection of several powerful, long-term market tailwinds, particularly the shift to complex biologics and self-administered therapies.
Business Model Scalability
High
Capital-intensive with high fixed costs for manufacturing facilities and R&D, but achieves significant economies of scale at high utilization rates, leading to margin expansion.
High. Once a component is designed into a blockbuster drug, it generates a long-term, high-margin revenue stream with relatively low incremental sales and marketing costs.
Scalability Constraints
- •
Long lead times for building and validating new manufacturing capacity.
- •
Global supply chain complexity and potential for disruptions.
- •
Requirement for significant, ongoing capital expenditure to meet growing demand and technological shifts.
- •
Maintaining stringent quality control and regulatory compliance across a growing global footprint of 50 sites.
Team Readiness
Strong. The leadership team demonstrates a clear strategy focused on high-growth markets (biologics, GLP-1s) and margin expansion through operational efficiency.
Mature and well-suited for its industry, with distinct Proprietary Products and Contract-Manufactured Products segments allowing for focused execution.
Key Capability Gaps
- •
Deep expertise in cryogenics and materials science specific to cell and gene therapy packaging.
- •
Digital capabilities to create integrated platforms for co-development and supply chain management with smaller biotech partners.
- •
Talent in sustainable materials science and circular economy business models.
Growth Engine
Acquisition Channels
- Channel:
Direct Enterprise Sales & Key Account Management
Effectiveness:High
Optimization Potential:Medium
Recommendation:Deepen partnerships by co-developing custom solutions for blockbuster drugs. Create a specialized team focused on the unique needs of emerging biotech companies to capture them early in their development cycle.
- Channel:
Thought Leadership & Content (Knowledge Center)
Effectiveness:High
Optimization Potential:High
Recommendation:Develop targeted content and webinars on navigating complex regulatory changes (like EU GMP Annex 1) and packaging challenges for new modalities like CGT and mRNA, solidifying West's position as an indispensable scientific partner.
- Channel:
Industry Reputation & Referrals
Effectiveness:High
Optimization Potential:Low
Recommendation:Maintain market leadership in quality and reliability, as reputation is a primary driver in this risk-averse industry. Actively cultivate case studies with successful emerging pharma partners.
- Channel:
Online Store (for small-scale orders)
Effectiveness:Medium
Optimization Potential:High
Recommendation:Expand product selection and create bundled 'R&D Starter Kits' for specific applications (e.g., pre-clinical biologic formulation testing) to become the default choice for early-stage companies.
Customer Journey
Long and complex, involving deep technical evaluation, regulatory consultation, and co-development, often spanning years from initial contact to commercial supply.
Friction Points
- •
Potentially slow onboarding and procurement processes for small, fast-moving biotech startups.
- •
Lack of flexible, small-batch solutions for early-stage R&D.
- •
Navigating West's extensive product catalog can be daunting for new customers without direct support.
Journey Enhancement Priorities
{'area': 'Early-Stage Biotech Engagement', 'recommendation': "Launch a 'Biotech Fast-Track' program with a dedicated concierge, simplified contracting, and pre-packaged R&D kits available through the online store."}
{'area': 'Digital Customer Portal', 'recommendation': "Enhance the 'My Account' portal into a comprehensive collaboration hub for tracking joint projects, accessing regulatory documentation on-demand, and managing forecasts."}
Retention Mechanisms
- Mechanism:
Regulatory Lock-In
Effectiveness:Very High
Improvement Opportunity:Proactively provide regulatory support and documentation to clients, making the process smoother and further cementing the partnership.
- Mechanism:
Deep Supply Chain Integration
Effectiveness:Very High
Improvement Opportunity:Offer advanced services like vendor-managed inventory and joint demand forecasting to become even more embedded in customer operations.
- Mechanism:
Long-Term Supply Agreements
Effectiveness:High
Improvement Opportunity:Develop innovative contract structures that offer flexibility for clinical development phases while securing long-term commercial supply.
Revenue Economics
Extremely Favorable. The value of ensuring the safety and efficacy of a multi-billion dollar drug far outweighs the cost of West's components, allowing for strong pricing power and high gross margins (35.7% in Q2 2025).
Exceptionally High (not publicly calculated). Customer acquisition cost is significant but amortized over decades of high-margin revenue from successful drugs.
High. Strong revenue growth (9.2% YoY in Q2 2025) and a high return on equity (17-18%) indicate efficient conversion of investment into revenue.
Optimization Recommendations
- •
Continue shifting the product mix toward high-value products (HVP) like NovaPure® and self-injection devices, which command premium pricing.
- •
Implement price optimization strategies based on the value delivered, particularly for components used in high-cost biologic and cell therapies.
- •
Introduce value-added analytical and regulatory services as a separate, recurring revenue stream.
Scale Barriers
Technical Limitations
- Limitation:
Innovation for Next-Generation Therapies
Impact:High
Solution Approach:Aggressively invest in R&D and strategic acquisitions/partnerships focused on packaging for cell & gene therapies, mRNA vaccines, and other novel modalities requiring specialized materials and cryogenic storage.
Operational Bottlenecks
- Bottleneck:
Manufacturing Capacity Expansion
Growth Impact:Could constrain ability to meet surging demand, especially for GLP-1 related products.
Resolution Strategy:Continue strategic capital expenditures on advanced manufacturing, focusing on automation and efficiency to maximize output from existing and new facilities.
- Bottleneck:
Global Supply Chain Resilience
Growth Impact:Vulnerability to geopolitical events, trade disputes (e.g., tariffs), and raw material shortages could disrupt production.
Resolution Strategy:Diversify raw material sourcing, increase regional manufacturing capabilities, and implement advanced supply chain monitoring and planning systems.
Market Penetration Challenges
- Challenge:
Competition from established players
Severity:Major
Mitigation Strategy:Compete on innovation, quality, and regulatory expertise rather than price. Key competitors include Gerresheimer, Schott, Datwyler, and Aptar. Focus on integrated systems (primary packaging + delivery device) to create a stronger competitive moat.
- Challenge:
Penetrating the emerging biotech/CGT sector
Severity:Major
Mitigation Strategy:Create a dedicated business unit or program with flexible offerings, faster turnaround times, and expert consultation tailored to the needs of smaller, R&D-intensive companies.
Resource Limitations
Talent Gaps
- •
Material scientists with expertise in polymers suitable for cryogenic storage.
- •
Experts in connected devices and software for 'smart' injector platforms.
- •
Business development and technical sales specialists with deep knowledge of the cell and gene therapy space.
High and ongoing. Sustained growth requires significant capital expenditure ($377M planned for 2024) for facility expansion and technology upgrades.
Infrastructure Needs
- •
Expanded manufacturing capacity for high-value prefillable syringes and self-injection devices.
- •
New R&D and analytical lab capabilities dedicated to cell and gene therapy packaging solutions.
- •
Enhanced digital infrastructure to support a seamless customer portal and advanced supply chain analytics.
Growth Opportunities
Market Expansion
- Expansion Vector:
Deeper Penetration into Biologics and GLP-1 Markets
Potential Impact:High
Implementation Complexity:Medium
Recommended Approach:Become the co-development partner of choice for these therapies by offering an integrated system of high-performance containment (e.g., Crystal Zenith vials) and delivery (e.g., auto-injectors). Double down on HVP capacity expansion.
- Expansion Vector:
Emerging Markets (Asia-Pacific)
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Establish/expand manufacturing and technical support hubs in key APAC biotech clusters to serve the rapidly growing regional pharmaceutical industry.
- Expansion Vector:
Dedicated Focus on Cell & Gene Therapy (CGT) Market
Potential Impact:High
Implementation Complexity:High
Recommended Approach:Develop a portfolio of products and services specifically for CGT, addressing challenges like cryogenic storage and handling. Pursue acquisitions of or partnerships with specialists in this niche.
Product Opportunities
- Opportunity:
Integrated 'Smart' Self-Injection Devices
Market Demand Evidence:Trend towards connected health, patient adherence monitoring, and demand for data from clinical trials.
Strategic Fit:High
Development Recommendation:Acquire or partner with a medical device technology firm to integrate connectivity (Bluetooth, NFC) and software into next-generation self-injection platforms.
- Opportunity:
Cryogenic Containment Solutions for CGT
Market Demand Evidence:The CGT packaging market is growing at a CAGR of ~11.8% and requires novel solutions for extreme temperatures.
Strategic Fit:High
Development Recommendation:Launch a dedicated R&D initiative focused on developing and validating new polymer and vial technologies capable of withstanding cryogenic shock and maintaining sterility for cell therapies.
- Opportunity:
Sustainable Packaging Portfolio
Market Demand Evidence:Increasing ESG pressure on major pharmaceutical clients and growing global regulations on plastics and waste.
Strategic Fit:Medium
Development Recommendation:Establish a 'Green Products' roadmap. Invest in R&D for medical-grade recyclable polymers and create a vial/component recycling pilot program with a major partner.
Channel Diversification
- Channel:
Value-Added Services (Analytical & Regulatory Consulting)
Fit Assessment:High
Implementation Strategy:Formalize existing expertise into a paid service offering. Market these services to small and mid-sized pharma companies that lack large in-house analytical and regulatory teams.
- Channel:
Strategic Co-development Platforms
Fit Assessment:High
Implementation Strategy:Build a digital platform that enables seamless collaboration between West's engineers and a client's drug development team, from concept through to commercialization.
Strategic Partnerships
- Partnership Type:
Technology Acquisition/Licensing
Potential Partners
- •
Startups in connected/smart device technology.
- •
Companies with novel polymer science for cryogenic applications.
- •
Firms specializing in AI for predictive maintenance in manufacturing.
Expected Benefits:Accelerate entry into new high-tech product areas and enhance manufacturing efficiency.
- Partnership Type:
Alliance with Contract Development and Manufacturing Organizations (CDMOs)
Potential Partners
- •
Lonza
- •
Catalent
- •
Thermo Fisher Scientific (Patheon)
Expected Benefits:Become the preferred primary packaging partner for CDMOs, gaining access to a wide portfolio of their early-stage biotech clients.
- Partnership Type:
Joint Venture/Equity Stake
Potential Partners
Daikyo Seiko, Ltd. (deepen existing partnership)
Innovators in cell therapy logistics and cryopreservation.
Expected Benefits:Secure access to critical technologies and manufacturing capabilities, particularly in high-growth Asian markets and the CGT space.
Growth Strategy
North Star Metric
Annual Revenue from High-Value Products (HVP) in Biologics, GLP-1, and CGT applications
This metric aligns directly with the most significant market tailwinds, focuses on the highest-margin segments, and measures success in capturing future growth. It moves beyond simple volume to track value creation.
Achieve 15-20% year-over-year growth in this specific revenue segment.
Growth Model
Innovation & Enterprise Partnership-Led Growth
Key Drivers
- •
Deep, long-term co-development partnerships with top-tier pharmaceutical clients.
- •
Continuous R&D to launch innovative products that address new therapeutic modalities.
- •
Superior quality and regulatory expertise that creates high switching costs.
- •
Strategic M&A to acquire new technologies and capabilities.
Structure business development and R&D into teams aligned with high-growth therapeutic areas (e.g., Biologics, CGT). Empower these teams to build deep relationships and act as consultants, not just suppliers.
Prioritized Initiatives
- Initiative:
Launch 'West CGT Solutions' Business Unit
Expected Impact:High
Implementation Effort:High
Timeframe:18-24 months
First Steps:Form a cross-functional task force to map the CGT market needs. Identify a leader for the new unit. Allocate dedicated R&D budget and explore a small, strategic acquisition of a company with relevant technology.
- Initiative:
Expand HVP Manufacturing Capacity for GLP-1 & Biologics
Expected Impact:High
Implementation Effort:High
Timeframe:24-36 months
First Steps:Finalize site selection for a new manufacturing facility focused on self-injection devices and prefillable syringe components. Secure long-term supply agreements with key customers to de-risk the investment.
- Initiative:
Enhance the Digital 'Biotech Fast-Track' Program
Expected Impact:Medium
Implementation Effort:Medium
Timeframe:6-9 months
First Steps:Expand the online store catalog with R&D-focused product bundles. Develop a streamlined digital onboarding process. Hire dedicated technical support specialists for this customer segment.
Experimentation Plan
High Leverage Tests
- Test Name:
CGT Packaging Pilot Program
Hypothesis:Offering a bundled solution of novel cryogenic vials and dedicated analytical support will significantly accelerate adoption by cell therapy startups.
Key Metrics:Number of pilot program participants, conversion rate to commercial supply agreement, customer satisfaction (NPS).
- Test Name:
Subscription-Based R&D Kits
Hypothesis:A subscription model for R&D kits via the online store will increase early-stage customer loyalty and provide predictable recurring revenue.
Key Metrics:Number of subscribers, monthly recurring revenue (MRR), churn rate.
Use a combination of leading indicators (e.g., number of co-development projects initiated, sample requests) and lagging indicators (e.g., revenue from new products, market share in target segments) tracked via a centralized growth dashboard.
Quarterly review of strategic pilots and business development initiatives. Monthly tracking of digital channel performance.
Growth Team
Maintain the core enterprise sales structure but create a dedicated, agile 'Growth Ventures' team.
Key Roles
- •
Head of Growth Ventures (reports to Chief Strategy Officer)
- •
Business Development Manager, Cell & Gene Therapy
- •
Product Manager, Digital Customer Experience & e-commerce
- •
Market Intelligence Analyst, Emerging Technologies
Build capabilities through a mix of internal training, hiring experts from the biotech and med-tech industries, and strategic acquisitions. Foster a culture that allows for calculated risks and faster decision-making within the Growth Ventures unit.
West Pharmaceutical Services is in an exceptionally strong position for sustained, long-term growth. Its foundation is built on a century of market leadership, deep customer integration, and a business model protected by high regulatory barriers. The company is perfectly aligned with the pharmaceutical industry's most powerful tailwinds: the shift to complex biologics, the explosive growth of GLP-1 drugs for obesity and diabetes, and the rise of self-administered therapies. These trends are fueling demand for West's high-value products (HVP), driving both revenue growth and margin expansion.
The primary growth engine is a sophisticated, partnership-led enterprise sales model. However, to accelerate growth, West must become more agile in capturing the next generation of therapies. The most significant opportunities lie in three key areas: 1) Dominating the packaging and delivery systems for biologics and GLP-1s through integrated, high-performance solutions; 2) Winning the emerging, high-growth Cell & Gene Therapy (CGT) market by developing novel cryogenic containment technologies; and 3) Better serving the fast-moving biotech startup ecosystem through a more agile, digitally-enabled service model.
Key barriers to scale are not market demand but operational: the ability to expand manufacturing capacity fast enough to meet demand, the need for continuous innovation to keep pace with new drug modalities, and navigating global supply chain complexities. The recommended strategy is to sharpen the company's focus on its most promising growth vectors. This involves establishing a dedicated business unit for CGT, doubling down on capital investment in HVP manufacturing, and enhancing the digital channels to capture early-stage customers. By defining its North Star Metric as 'Revenue from HVPs in high-growth therapeutic areas,' West can ensure the entire organization is aligned on creating maximum value in the future of injectable medicines.
Legal Compliance
West Pharmaceutical Services maintains a comprehensive and accessible Privacy Policy. It clearly outlines the types of personal data collected (e.g., identity, contact, professional details, payment information), the purposes for processing (e.g., customer service, marketing, order fulfillment), and the conditions for data sharing (e.g., with corporate affiliates, service providers, or for legal reasons). The policy addresses international data transfers, noting that data may be moved to countries with different levels of data protection, but mentions the use of Standard Contractual Clauses (SCCs) to govern these transfers. It also specifies user rights, including access, correction, removal, and objection to processing, providing contact information for exercising these rights. The policy acknowledges the collection of data from children under 13 is not done knowingly. It specifically references a wide array of international data protection regulations, demonstrating a strong awareness of its global compliance obligations.
The website offers specific Terms of Sale for its online store and separate Terms of Service for its 'DeltaCube™' web-based platform. The Terms of Sale are robust for a B2B context, covering critical commercial aspects like order acceptance, pricing validity (30 days), delivery terms (EXW Incoterms 2020), and limitations on refunds. Crucially, they include a 'Limitation on End Usage' clause, restricting the use of their products to medical and pharmaceutical applications and prohibiting resale outside of incorporation into final drug products. The terms also effectively limit West's liability regarding buyer-provided materials and product modifications. The DeltaCube™ platform terms are equally detailed, covering account creation, access rights, and intellectual property. However, a general 'Terms of Use' document governing the entire website's informational content (like the Knowledge Center and blogs) was not immediately apparent, which could be a minor gap.
Upon visiting the website, a cookie consent banner is prominently displayed. It provides users with 'Accept All Cookies' and 'Reject All' options, which is a strong practice for GDPR compliance. It also includes a 'Cookie Settings' option, allowing for granular control over different categories of cookies (e.g., Strictly Necessary, Functional, Performance, Targeting). This level of user control and transparency is excellent. The initial state of the toggles for non-essential cookies is 'off,' requiring an affirmative action from the user to opt-in, which aligns with best practices for privacy regulations like GDPR.
West's overall data protection posture is strong, reflecting the seriousness of its industry and global operations. The company has a dedicated Chief Compliance & Privacy Officer. The Privacy Policy is detailed and covers key requirements of major global regulations. The implementation of a granular and user-friendly cookie consent mechanism further strengthens this position. The company explicitly states its commitment to securing personal data and maintaining adherence to privacy laws in all jurisdictions where it operates. The mention of SCCs for data transfers shows a mature approach to handling the complexities of global data flows. The separate, detailed privacy notice for their 'WestGives' platform also demonstrates a thorough, context-specific approach to data protection.
A high-level review of the website suggests a good-faith effort towards accessibility, but reveals potential gaps. The site uses clear fonts and has good color contrast on its main pages. Navigation appears logical. However, a deeper look at the code reveals some potential issues. For example, the carousel controls ‹ and › are implemented as links with href="#" which may not be optimal for screen reader users if not properly labeled with ARIA attributes. A full WCAG 2.1 AA audit would be necessary to identify all non-compliance issues. Given the global nature of the business and legal precedents holding B2B websites to ADA standards in the US, achieving formal WCAG 2.1 AA compliance is a strategic imperative to avoid legal risk and ensure access for all potential business partners.
As a critical supplier to the pharmaceutical and biotechnology industries, West's legal positioning is heavily influenced by regulations from bodies like the FDA and EMA. The website content strategically positions West as a regulatory-savvy partner. The 'Regulatory Support' section is a significant asset, detailing how West helps clients with Drug Master Files (DMFs), compliance certifications (e.g., for materials of animal origin), and navigating global registration strategies. This proactively addresses customer concerns about supply chain integrity and compliance with Current Good Manufacturing Practices (cGMP). As a publicly-traded company (NYSE: WST), West is also subject to SEC regulations. The investor relations section of their site must comply with disclosure rules, and any forward-looking statements made on the site require a 'safe harbor' disclaimer to mitigate securities litigation risk, a risk highlighted by a recent securities class action lawsuit filed against the company.
Compliance Gaps
- •
Absence of a readily accessible, general 'Terms of Use' document for the informational sections of the website (blogs, Knowledge Center), which could leave ambiguity around IP rights and liability for informational content.
- •
No explicit accessibility statement or policy is visible on the site, which could indicate a lack of a formal, ongoing commitment to WCAG standards.
- •
The investor relations section lacks a prominent, easily identifiable 'Safe Harbor' statement for forward-looking information on its main pages, increasing risk related to SEC compliance.
Compliance Strengths
- •
A comprehensive, globally-aware Privacy Policy that addresses multiple international regulations and user rights.
- •
Excellent implementation of a cookie consent banner with clear 'Accept All', 'Reject All', and granular 'Cookie Settings' options.
- •
Strong, industry-specific Terms of Sale for their online store that clearly define usage limitations and liability.
- •
Proactive and strategic use of website content (e.g., 'Regulatory Support' section) to demonstrate deep industry compliance knowledge (FDA, EMA) and build customer trust.
Risk Assessment
- Risk Area:
SEC Compliance & Investor Relations
Severity:High
Recommendation:Immediately add a clear 'Safe Harbor' statement to the main investor relations page and any press releases. This statement should caution that forward-looking statements involve risks and uncertainties and are not guarantees of future performance, referencing SEC filings for more details. This is critical given recent securities litigation.
- Risk Area:
Website Accessibility (ADA/WCAG)
Severity:Medium
Recommendation:Commission a formal WCAG 2.1 AA audit of the entire website. Publish an 'Accessibility Statement' on the site, outlining the commitment to accessibility and providing a contact method for users who encounter barriers. Remediate issues identified in the audit, starting with the most critical ones.
- Risk Area:
General Website Liability
Severity:Low
Recommendation:Draft and publish a general 'Terms of Use' policy, accessible from the website footer. This policy should cover permissible use of website content, disclaimers of warranties for informational content (e.g., in the Knowledge Center), intellectual property rights, and limitations of liability.
High Priority Recommendations
- •
Add a prominent 'Safe Harbor' disclaimer for forward-looking statements in the investor relations section to mitigate securities litigation risk.
- •
Initiate a formal WCAG 2.1 AA accessibility audit and remediation plan to ensure compliance with ADA and other global accessibility standards, reducing legal exposure.
- •
Develop and link a general 'Terms of Use' document in the website footer to govern the use of informational content and limit liability.
Overall, West Pharmaceutical Services demonstrates a sophisticated and mature legal compliance posture, which it effectively leverages as a strategic asset. The company's digital presence is clearly designed to build trust in the highly regulated pharmaceutical supply chain. The robust data privacy framework, evidenced by a detailed global policy and a best-in-class cookie banner, is a significant strength that facilitates market access in jurisdictions with stringent privacy laws like the EU. Furthermore, the website's content, particularly the 'Regulatory Support' section, is a masterclass in strategic legal positioning. It transforms compliance from a cost center into a competitive advantage by showcasing expertise in FDA and EMA regulations, thereby reassuring B2B customers about supply chain integrity.
However, there are notable areas for improvement. The most critical risk lies in SEC compliance; the absence of a readily visible 'Safe Harbor' statement for forward-looking information creates unnecessary exposure to securities litigation, especially in light of recent lawsuits. Secondly, while the site has some accessible features, the lack of a formal accessibility statement and potential technical gaps present a medium-level legal risk under the ADA and other accessibility mandates. Addressing these gaps is not merely about mitigating risk but about reinforcing the corporate image of quality and diligence. By tightening its SEC disclosures and formalizing its commitment to web accessibility, West can further solidify its position as a trusted, legally resilient market leader.
Visual
Design System
Corporate Professional
Good
Developing
User Experience
Navigation
Horizontal Mega Menu (Desktop), Hamburger (Mobile)
Clear
Good
Information Architecture
Logical
Somewhat clear
Moderate
Conversion Elements
- Element:
Main Header CTA 'Sign In'
Prominence:Medium
Effectiveness:Somewhat effective
Improvement:Increase visual weight. Change from a simple link to a ghost button or a solid, low-emphasis color button to differentiate it from informational links.
- Element:
Homepage 'Learn More' Buttons
Prominence:Medium
Effectiveness:Somewhat effective
Improvement:These CTAs are styled as secondary (ghost buttons), which diminishes their visual importance. The primary CTA on a page should be a solid color (e.g., the brand's green) to draw the user's eye and encourage clicks.
- Element:
Create My Account' CTA
Prominence:Medium
Effectiveness:Somewhat effective
Improvement:The 'Register Here' link is visually weak. It should be a button with a clear action-oriented label like 'Create My Account' to improve its visibility and conversion potential.
- Element:
Knowledge Center & Press Release CTAs
Prominence:High
Effectiveness:Effective
Improvement:The use of a contrasting dark teal background for the Press Releases section is effective. This visual treatment should be considered for other key conversion sections to guide user attention.
Assessment
Strengths
- Aspect:
Professional and Clean Aesthetic
Impact:High
Description:The website projects a credible, professional, and trustworthy image, which is critical in the B2B pharmaceutical and biotech industry. The use of high-quality imagery, ample white space, and a consistent color palette reinforces West's position as a market leader.
- Aspect:
Clear Information Architecture at Top Level
Impact:High
Description:The main navigation menu is logically structured around key user needs: 'Products', 'Services and Solutions', 'Challenges', and 'Support'. This makes it relatively easy for users to find the primary section relevant to their goals.
- Aspect:
High-Quality, Relevant Imagery
Impact:Medium
Description:The photography used throughout the site is professional and relevant, showing clean lab environments, manufacturing processes, and diverse teams. This helps to build trust and visually communicate the company's expertise and values.
Weaknesses
- Aspect:
Inconsistent CTA Hierarchy
Impact:High
Description:There is a lack of clear visual distinction between primary, secondary, and tertiary calls-to-action. Most CTAs are styled as ghost buttons or simple links, reducing their prominence and potentially lowering engagement on key conversion paths like account creation or contacting sales.
- Aspect:
Dense Text and Content Overload
Impact:Medium
Description:Several sections, particularly on interior pages, feature long paragraphs and dense blocks of text. This increases cognitive load and makes it difficult for users to quickly scan and digest information. Key insights and data points are not visually emphasized.
- Aspect:
Underutilized Visual Storytelling
Impact:Medium
Description:While the imagery is good, there's an opportunity to use visuals more dynamically. For example, using diagrams, infographics, or interactive elements to explain complex processes or product benefits would be more engaging than text-heavy descriptions.
- Aspect:
Generic Card-Based Layouts
Impact:Low
Description:The homepage relies heavily on a generic grid of cards. While organized, this layout is common and does little to create a unique or memorable brand experience. Varying the layout with full-width sections, asymmetrical designs, or more dynamic visuals could enhance engagement.
Priority Recommendations
- Recommendation:
Establish a Clear CTA Visual Hierarchy
Effort Level:Low
Impact Potential:High
Rationale:Define distinct styles for primary (solid color fill), secondary (ghost button), and tertiary (link) actions. Apply this system consistently across the site. This will guide users toward key conversion goals, such as 'Contact Us' or 'Explore Products', significantly boosting lead generation and user flow efficiency.
- Recommendation:
Break Up Dense Content with Visual Aids
Effort Level:Medium
Impact Potential:High
Rationale:Incorporate icons, mini-infographics, accordions for FAQs, and tabbed content sections to make complex information more digestible. This will reduce cognitive load, improve comprehension of West's value propositions, and keep users engaged for longer.
- Recommendation:
Enhance Visual Storytelling in Key Sections
Effort Level:Medium
Impact Potential:Medium
Rationale:For sections like 'Company Background' and 'Our Values', replace static images and text blocks with more dynamic elements. Consider a timeline graphic for company history or short video testimonials. This will create a more compelling brand narrative and stronger emotional connection with potential partners and employees.
- Recommendation:
Refine Homepage Layout for Better Guidance
Effort Level:Low
Impact Potential:Medium
Rationale:Revise the homepage layout to better guide different user personas (e.g., researcher, procurement manager). Create clearer pathways for each audience by using distinct headlines and visually differentiated section backgrounds. This helps key audiences self-segment and find relevant information faster.
Mobile Responsiveness
Good
The design adapts well to standard tablet and mobile breakpoints. Content stacks logically, and navigation collapses into an accessible hamburger menu.
Mobile Specific Issues
Long scrolling required due to the stacking of multi-column desktop layouts, especially in the footer.
Some text links in dense paragraphs can be small and difficult to tap accurately on smaller screens.
Desktop Specific Issues
Large hero images on desktop can push key content and CTAs below the fold on certain screen resolutions.
The expansive footer, while comprehensive, can feel overwhelming and visually cluttered on large monitors.
Overall Strategic Assessment
West Pharma's website successfully projects a professional, credible, and corporate image befitting a global leader in pharmaceutical packaging and delivery systems. The design is clean, the color palette is appropriately conservative, and the information architecture is logical at a high level. For its target audience of pharmaceutical companies, researchers, and medical device firms, the site serves as a trustworthy and comprehensive resource.
However, the user experience is hampered by a lack of clear visual hierarchy and persuasive design, particularly concerning calls-to-action (CTAs). The design system, while consistent in its use of color and typography, is in a 'developing' stage; it lacks the maturity to effectively guide users toward key conversion goals. The overall impression is more of a digital brochure than a strategic tool for lead generation and user engagement.
Detailed Analysis
-
Design System & Brand Identity:
- The visual style is clean and corporate, utilizing a palette of light grays, whites, and brand-accent colors (green and teal). This aligns well with the brand's positioning as a scientific and reliable partner.
- Brand consistency is good. The logo, color palette, and typography are applied consistently across the pages shown.
- The system's maturity is 'Developing'. While basic components are consistent, there's a lack of nuanced application. For example, all button styles are treated with similar low visual weight, failing to differentiate primary actions from secondary ones.
-
Visual Hierarchy & Information Architecture:
- The homepage effectively communicates the core value proposition—"Who We Are"—immediately. The hierarchy on the page flows from brand identity to product categories, company background, and corporate values.
- The use of card-based layouts organizes content neatly but also creates a visual monotony. Sections lack distinct visual treatments to draw attention to priority areas, like the 'Online Store' or 'Knowledge Center'.
- The cognitive load is moderate. While navigation is clear, the dense blocks of text on interior pages (e.g., 'About West') require significant user effort to parse.
-
Navigation & User Flow:
- The primary navigation uses a horizontal mega-menu on desktop, which is a standard and effective pattern for organizing a large amount of content. It cleanly collapses into a hamburger menu on mobile.
- User flow clarity is somewhat clear. While a user can navigate to a top-level section easily, the journey within that section can be unclear. The overuse of similar-looking 'Learn More' buttons doesn't effectively guide the user on the most important path.
-
Mobile Responsiveness:
- The site demonstrates a solid responsive design foundation. Layouts reflow logically, images scale correctly, and text remains legible. The mobile experience is functional and does not present major usability barriers.
- The primary issue is the length of pages on mobile due to the simple stacking of columns, which can lead to user fatigue.
-
Visual Conversion Elements & CTAs:
- This is the most significant area of weakness. The site fails to create compelling conversion points. Key actions like 'Sign In' or 'Register Here' are presented as simple text links. The primary CTAs within content sections are ghost buttons, which studies show typically have lower conversion rates than solid-filled buttons because they are perceived as less important.
- There is no clear visual language to tell a user, "This is the most important action to take on this page." This lack of direction likely leads to lower engagement and fewer conversions (e.g., account creations, contact form submissions).
-
Visual Storytelling & Content Presentation:
- The website tells the 'what' effectively (what West does) but struggles to tell the 'why' (why a customer should partner with them). The content about culture, giving, and careers is presented in the same static format as product information.
- There is a missed opportunity to use more engaging content formats like video, interactive diagrams of their systems, or compelling case study narratives that mix imagery, data callouts, and testimonials.
Discoverability
Market Visibility Assessment
West Pharma is a well-established leader in the injectable drug containment and delivery market, and its digital presence reflects this through a strong emphasis on its 'Knowledge Center' and technical documentation. They have a solid foundation for brand authority, particularly around regulatory topics like EU GMP Annex 1, where they actively publish content to guide customers. However, their authority is primarily product-centric. Competitors like SCHOTT Pharma are building educational platforms like 'Pharmaversity®' to cultivate a broader expert community, creating a potential vulnerability for West. While West is seen as a reliable manufacturer, there is an opportunity to elevate its brand to a visionary, forward-looking scientific partner.
West's digital presence aligns with its position as a major player in the injectable drug delivery market. They appear in financial analyses and market reports as a key competitor alongside companies like Gerresheimer, SCHOTT Pharma, and BD. However, their visibility for high-growth, trend-based keywords (e.g., 'sustainable pharmaceutical packaging', 'GLP-1 drug delivery devices') is less prominent than their core product terms. Competitors like Gerresheimer are aggressively marketing their role in the GLP-1 market, potentially capturing greater digital mindshare in this high-growth segment. West's visibility seems to mirror its current market share rather than proactively capturing future growth areas.
The website is well-structured for customer acquisition from its core B2B audience (pharmaceutical and biotech companies). Clear pathways exist to product information, technical documentation via the 'Knowledge Center', and an 'Online Store' for small-quantity orders. This effectively serves engineers and procurement specialists already familiar with West or seeking specific components. The primary opportunity for improvement lies in capturing potential customers earlier in their journey—during the research and development phase when they are exploring solutions to complex formulation or delivery challenges, not just searching for a specific vial or stopper.
West's website communicates its global footprint with mentions of 50 sites worldwide. The content, such as the announcement of a new lab in Germany, reinforces its presence in key markets like Europe. However, the digital experience itself is not heavily localized. While the company has a strong physical presence in regions like North America (45% of revenue) and internationally (55%), the digital content does not appear to be strategically tailored or translated to capture specific regional market opportunities or address local regulatory nuances beyond major topics like EU GMP. Competitors like SCHOTT Pharma explicitly list their numerous international sites, potentially creating a stronger impression of local partnership for regional prospects.
West demonstrates deep expertise in its core domains: vial containment, prefillable systems, and self-injection devices. Their 'Knowledge Center' is a significant asset, covering technical and regulatory topics thoroughly. However, there are strategic gaps in emerging areas that are becoming critical decision factors for customers. These include sustainability in primary packaging, advanced delivery systems for biologics and cell/gene therapies, and digitalization in drug delivery (connected devices). While they cover current needs well, their content does not fully address the next generation of industry challenges, which competitors are beginning to explore.
Strategic Content Positioning
West's content is strongest at the consideration and decision stages of the B2B customer journey. Engineers and scientists can find detailed product specifications and technical documents, while procurement managers can use the online store or contact sales. The weakness is in the initial 'Awareness' stage. A researcher grappling with a novel biologic's stability issues may not find high-level, problem-oriented content that leads them to West's solutions. The content assumes a certain level of customer knowledge, potentially missing the opportunity to educate and capture prospects at the very beginning of their discovery process.
Significant thought leadership opportunities exist in positioning West as a partner in solving future pharmaceutical challenges. Key areas include: 1) Sustainability: The industry is actively seeking PVC-free and more recyclable packaging solutions. West could lead the conversation on circularity in primary packaging. 2) Biologics & Advanced Therapies: These complex molecules require innovative containment solutions. Content dedicated to solving challenges for mRNA, cell, and gene therapies would establish authority. 3) Digital Health: As competitors like Gerresheimer explore connected devices and digital therapy support, West has an opportunity to publish its vision for the future of patient-centric, data-driven drug delivery.
Competitors are successfully leveraging different content strategies. SCHOTT Pharma has created a branded educational ecosystem ('Pharmaversity®') that fosters a community and positions them as educators, not just suppliers. Gerresheimer is very vocal about their role in the high-growth GLP-1 market and their focus on innovation and digitalization. The primary gap for West is high-level, forward-looking content. They excel at the 'how' (technical specifications) but could improve on the 'why' and 'what's next' (strategic industry trends, future challenges), which would attract a more senior, strategic audience within their customer base.
The brand message of being 'By Your Side for a Healthier World™' and focusing on quality and partnership is consistently reflected across the website. The tone is professional, reliable, and patient-focused. This messaging is effectively reinforced through press releases about awards and content on quality standards like EU GMP Annex 1. The visual identity and corporate voice are consistent, projecting an image of a stable, trustworthy market leader.
Digital Market Strategy
Market Expansion Opportunities
- •
Target the Biologics/Advanced Therapies Sector: Develop a dedicated content hub with white papers, case studies, and webinars addressing the unique containment and delivery challenges of cell therapies, gene therapies, and other large molecules.
- •
Expand into High-Growth Therapeutic Areas: Create targeted campaigns and content for rapidly growing drug classes, such as GLP-1 agonists for obesity and diabetes, to capture the significant market attention currently focused there.
- •
Lead the Sustainability Conversation: Launch an initiative focused on sustainable primary packaging solutions, including research reports, material analyses, and partnerships, to attract environmentally conscious pharmaceutical companies.
Customer Acquisition Optimization
- •
Develop Problem-Oriented Content: Create content that addresses the high-level challenges of R&D scientists (e.g., 'How to ensure stability for sensitive biologics,' 'Mitigating interaction risk in prefilled syringes') to attract them during the early research phase, before they are searching for specific components.
- •
Leverage the 'Knowledge Center' for Lead Generation: Gate premium, high-value content such as in-depth research reports, regulatory compliance guides, and on-demand webinars to convert expert traffic into qualified leads.
- •
Enhance Digital Experience for Key Segments: Create interactive tools or solution finders that guide users from a specific problem (e.g., drug viscosity, dosage requirements) to the ideal West product and service combination, simplifying the complex decision-making process.
Brand Authority Initiatives
- •
Launch a 'Future of Injectables' Report Series: Publish an annual flagship report featuring insights from West's experts and industry leaders on trends in drug delivery, manufacturing, and regulation.
- •
Promote Internal Experts: Feature West's scientists and engineers as thought leaders through bylined articles in industry publications, speaking engagements at major conferences, and a dedicated expert Q&A section on the blog.
- •
Host a Global Digital Summit: Organize a virtual conference focused on innovation in drug containment and delivery, bringing together customers, partners, and regulators to discuss the industry's most pressing challenges.
Competitive Positioning Improvements
- •
Shift Messaging from 'Component Supplier' to 'Integrated Solutions Partner': Emphasize the combined value of West's products and analytical services, highlighting how this integrated approach de-risks and accelerates drug development compared to sourcing components and services separately.
- •
Create Competitive Comparison Content: Develop content that subtly guides customers on key criteria for selecting a packaging partner, focusing on West's strengths like quality, regulatory support, and a comprehensive product portfolio without directly naming competitors.
- •
Highlight Innovation in Core Products: More prominently feature the R&D and technological advancements behind their products, moving the perception from a provider of commodities (stoppers, vials) to an innovator of high-performance systems.
Business Impact Assessment
Success will be measured by an increase in 'Share of Voice' for strategic, non-branded keywords related to biologics, sustainable packaging, and GLP-1 delivery systems. Growth in organic search traffic and rankings for these terms against key competitors (SCHOTT, Gerresheimer) will indicate expanding digital market share.
Key metrics include the number of qualified leads generated from gated content within the 'Knowledge Center,' sample requests originating from problem-oriented content, and an increase in inquiries for analytical services. Tracking the conversion rate from these digital touchpoints to sales opportunities will be critical.
Authority will be measured by the increase in organic backlinks from reputable industry domains, media mentions of West's research and experts, and invitations for West's personnel to speak at industry events. Growth in direct traffic and branded search volume also indicates rising brand authority and recall.
Benchmarking will involve tracking keyword ranking performance for high-value commercial terms (e.g., 'prefillable syringes,' 'self-injection devices') against a defined list of top competitors. The goal is to achieve and maintain a top-three ranking for critical product categories, demonstrating superior digital visibility.
Strategic Recommendations
High Impact Initiatives
- Initiative:
Develop a 'Biologics & Advanced Therapies' Content Hub
Business Impact:High
Market Opportunity:The biologics market is growing significantly faster than the overall pharmaceutical market. Positioning West as the go-to expert for the complex containment needs of these high-value drugs will capture significant market share and establish a long-term competitive advantage.
Success Metrics
- •
Number of qualified leads from the hub
- •
Organic search rankings for biologic-related keywords
- •
Engagement rates on white papers and webinars
- •
Inbound inquiries mentioning advanced therapies
- Initiative:
Launch a 'Sustainable Pharmaceutical Packaging' Leadership Program
Business Impact:Medium
Market Opportunity:Sustainability is a growing priority for pharmaceutical companies, driven by regulation and consumer demand. Proactively leading this conversation allows West to shape industry standards and become the preferred partner for eco-conscious drug manufacturers, turning a potential compliance issue into a competitive differentiator.
Success Metrics
- •
Media mentions related to sustainability
- •
Downloads of sustainability reports
- •
Partnership inquiries from eco-focused organizations
- •
Website traffic for sustainability-related keywords
- Initiative:
Create a 'Problem-to-Solution' Digital Experience
Business Impact:High
Market Opportunity:The B2B pharma customer journey is complex and often starts with a scientific or technical problem, not a product name. By creating content and tools that guide R&D professionals from their specific challenge (e.g., drug stability, particle contamination, usability) to an integrated West solution, the company can capture customers much earlier in the development lifecycle, increasing the likelihood of being specified in the final product.
Success Metrics
- •
Engagement with new solution-finder tools
- •
Increased conversion rates from blog/resource pages
- •
Reduction in website bounce rate
- •
Growth in early-stage R&D leads
Shift West Pharma's digital market position from a premier component manufacturer to an indispensable scientific and solutions partner for the entire lifecycle of injectable drug development. This involves moving beyond product specifications to address the strategic challenges of customers—from ensuring the stability of next-generation biologics to navigating complex regulations and meeting sustainability goals. The digital presence should serve as a platform for scientific exchange and problem-solving, solidifying West's role as an integral part of their customers' success.
Competitive Advantage Opportunities
- •
Leverage the synergy of 'Products + Services': West's unique ability to offer both high-quality components and comprehensive analytical services is a key differentiator. Digital content should consistently highlight how this integrated offering reduces risk, cost, and time-to-market for clients.
- •
Weaponize Regulatory Expertise: Transform deep regulatory knowledge (e.g., EU GMP Annex 1) from a product feature into a strategic asset. Create a dedicated regulatory intelligence hub to become the authoritative source for guidance, attracting customers who prioritize compliance and risk mitigation.
- •
Emphasize 100 Years of Trust and Data: Utilize the company's century-long history not just as a milestone but as evidence of unparalleled expertise and data. Create content that showcases insights derived from decades of experience, reinforcing the message of reliability and quality that newer competitors cannot match.
Digital Market Presence Analysis: West Pharmaceutical Services, Inc.
1. Executive Summary
West Pharmaceutical Services (westpharma.com) presents a strong and professional digital presence that accurately reflects its status as a global leader in injectable drug containment and delivery systems. The website effectively serves its core audience of engineers and procurement professionals in the later stages of the customer journey. Its key digital asset is the 'Knowledge Center,' which establishes credibility and provides essential technical documentation. However, a significant strategic opportunity exists to evolve the digital presence from that of a top-tier component supplier to an indispensable scientific and solutions partner. This involves capturing customers earlier in their research process, demonstrating thought leadership on future industry challenges, and more effectively competing for digital mindshare in high-growth segments like biologics and sustainable packaging.
2. Market Visibility Assessment
West commands strong visibility for its branded terms and core product categories, aligning with its established market position. The company is frequently cited in financial and market reports as a key player. Their authority is most pronounced on technical and regulatory topics, such as the EU GMP Annex 1 revision, where they have created valuable, guiding content. The primary visibility gap is in emerging, high-intent topic areas. Competitors like Gerresheimer are building strong digital narratives around their role in the booming GLP-1 market, while SCHOTT Pharma is investing in broad educational platforms like 'Pharmaversity®'. West's digital presence is currently more reactive to its market position than proactive in shaping its future one.
3. Strategic Content Positioning
The company's content is heavily weighted towards the consideration and decision phases of the B2B buyer's journey. It excels at providing the detailed specifications and quality assurances needed by an engineer ready to select a component. However, it is less effective at attracting a scientist in the early stages of drug development who is grappling with a broader challenge, such as formulation stability or container closure integrity for a sensitive biologic. The content assumes the user knows what they are looking for. Key opportunities lie in creating high-level, problem-oriented content that addresses future industry challenges, particularly in sustainability, advanced biologics, and digital health.
4. Strategic Recommendations
To secure and expand its market leadership in an evolving pharmaceutical landscape, West Pharma should pursue a three-pronged digital strategy:
-
Initiative 1: Dominate the Biologics & Advanced Therapies Conversation: The future of pharmaceuticals lies in complex biologics, and their containment is a critical challenge. By launching a dedicated digital hub with expert content (white papers, webinars, case studies) focused on solving these unique problems, West can position itself as the essential partner for this high-value market segment. This moves the brand from a supplier to a critical enabler of innovation.
-
Initiative 2: Shift from Product-First to Problem-First Content: Re-orient the content strategy to intercept customers at the beginning of their journey. Develop tools, guides, and articles that address the fundamental scientific problems their customers face. A 'Solution Finder' that guides a user from a challenge (e.g., 'preventing delamination') to an integrated West product-and-service package would be a powerful tool for early-stage customer acquisition.
-
Initiative 3: Champion an Area of Future Industry Importance: Proactively claim a thought leadership position in a crucial emerging area. Sustainability in primary packaging presents a perfect opportunity. By launching a leadership program with research reports and industry partnerships, West can transform a potential regulatory burden into a competitive advantage, attracting a new generation of pharma clients for whom sustainability is a key purchasing criterion.
5. Business Impact
By executing this strategy, West Pharmaceutical Services can achieve significant business outcomes. Attracting customers earlier in the R&D cycle will increase the probability of being specified in drug filings, creating long-term, sticky revenue streams. Establishing thought leadership in biologics and sustainability will not only build the brand's authority but also attract premium talent and strategic partnerships. Ultimately, this evolution of the digital presence will solidify West's competitive advantage, reduce customer acquisition costs for high-value segments, and ensure the company is positioned for leadership for the next 100 years.
Strategic Priorities
Strategic Priorities
- Title:
Launch 'West CGT Solutions' - A Dedicated Business Unit for Cell & Gene Therapy
Business Rationale:The Cell & Gene Therapy (CGT) market is a high-growth frontier with unique, complex packaging needs (e.g., cryogenic storage) that are not fully addressed by competitors. Establishing a dedicated unit will focus R&D, commercial, and operational resources to capture first-mover advantage and create a significant new revenue stream.
Strategic Impact:Positions West as the indispensable partner for next-generation medicines, moving beyond traditional injectables. This creates a powerful new growth engine and establishes a defensible moat in the most innovative segment of the pharmaceutical industry.
Success Metrics
- •
Annual revenue from CGT-specific products and services
- •
Number of CGT therapies in clinical trials using West components
- •
Market share within the CGT packaging and containment sector
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Market Expansion
- Title:
Establish a 'Biotech Fast-Track' Program to Win the Emerging Pharma Segment
Business Rationale:The analysis identified 'Emerging Drug Developers' as a high-potential segment but noted friction points (complexity, slow onboarding) in West's current model. A dedicated program with agile services, bundled R&D kits, and dedicated support will capture these innovative companies early, ensuring West is their partner from clinical trials to commercial success.
Strategic Impact:Accelerates customer acquisition in the fastest-growing part of the drug development ecosystem. By embedding West's components at the R&D stage, this initiative leverages the company's 'regulatory lock-in' advantage with the next generation of blockbuster drugs.
Success Metrics
- •
Number of new emerging biotech/pharma accounts acquired
- •
Revenue growth from the 'Online Store' and R&D kits
- •
Conversion rate from 'Fast-Track' program to long-term supply agreements
Priority Level:HIGH
Timeline:Quick Win (0-3 months)
Category:Customer Strategy
- Title:
Systematically Evolve from Component Supplier to 'Integrated De-Risking Partner'
Business Rationale:West's key advantage is its ability to combine high-quality products with deep scientific and regulatory expertise. The current brand messaging and service model do not fully monetize this. This initiative will realign sales, marketing, and service delivery to focus on selling a holistic solution that reduces client risk and accelerates their time-to-market.
Strategic Impact:Elevates the company's strategic importance to customers, shifting the conversation from component price to total lifecycle value. This strengthens pricing power, deepens customer relationships, and creates a durable competitive advantage over component-focused rivals.
Success Metrics
- •
Increase in revenue per customer from bundled product/service offerings
- •
Growth in standalone revenue from analytical and regulatory services
- •
Improved customer retention and Net Promoter Score (NPS)
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Brand Strategy
- Title:
Pioneer 'Smart Packaging' Data Services via Strategic Partnership or Acquisition
Business Rationale:The future of drug delivery is digital, involving connected devices that monitor adherence and ensure quality. Competitors are exploring this whitespace. Proactively building or acquiring a 'smart packaging' platform will create a new, high-margin, data-as-a-service (DaaS) revenue stream and an incredibly sticky customer ecosystem.
Strategic Impact:Transforms West from a physical product company into a data-enabled healthcare technology leader. This creates a powerful, recurring-revenue moat and provides invaluable real-world data to drive future innovation and deepen partnerships with pharmaceutical clients.
Success Metrics
- •
Number of strategic partnerships or acquisitions completed in the smart packaging space
- •
Launch of a pilot 'Smart Packaging' program with a key customer
- •
Development of a recurring revenue model for data services
Priority Level:MEDIUM
Timeline:Long-term Vision (12+ months)
Category:Revenue Model
- Title:
Become the Undisputed Leader in GLP-1 Drug Delivery Systems
Business Rationale:The GLP-1 market for diabetes and obesity is experiencing explosive, once-in-a-generation growth, creating massive demand for self-injection devices. The analysis shows this is a key growth driver. This initiative focuses investment and marketing to dominate the supply chain for this blockbuster drug class.
Strategic Impact:Captures a disproportionate share of the fastest-growing therapeutic area in the pharmaceutical industry. Solidifies West's market leadership in high-value delivery systems, driving significant revenue growth and margin expansion for the foreseeable future.
Success Metrics
- •
Revenue and market share specifically from GLP-1 related components
- •
Number of new long-term supply agreements with leading GLP-1 drug manufacturers
- •
Expansion of manufacturing capacity dedicated to self-injection devices
Priority Level:HIGH
Timeline:Strategic Initiative (3-12 months)
Category:Market Position
To secure its next century of market leadership, West must evolve from a world-class component manufacturer into an indispensable scientific solutions partner. This requires aggressively capturing the future of medicine by dominating the supply chain for next-generation therapies like GLP-1s and Cell & Gene Therapies, while simultaneously transforming its business model to better serve the agile needs of the emerging biotech ecosystem.
The key competitive advantage West must build is its position as the premier end-to-end de-risking partner for injectable medicines. This is achieved by uniquely integrating high-performance components, delivery systems, and expert analytical/regulatory services, shifting the customer focus from component price to total lifecycle value and speed-to-market.
The primary growth catalyst is the deep penetration and leadership in high-value, high-growth therapeutic areas, specifically the booming GLP-1 market and the nascent, complex Cell & Gene Therapy (CGT) sector. Offering specialized, integrated solutions for these markets will drive the majority of near- and long-term value creation.